Abstract
Toll-like receptor (TLR) signaling activation by pathogens is critical to the induction of immune responses, and demands tight regulation. We describe in this study that CC chemokine ligand 2 (CCL2) secretion triggered by TLR4 or TLR8 engagement is strongly inhibited upon simultaneous activation of both TLRs in human monocyte-derived dendritic cells (DCs). Impaired CCL2 secretion occurs concomitantly to interleukin-12 up-regulation, being part of a complex regulatory circuit ensuring optimal T helper type 1 polarization. Interestingly, triggering selected TLRs or their combinations differently affects nuclear factor-κB p65 activation and microRNA expression. Overall, these results indicate that CCL2 supplies an important immunomodulatory role to DCs, and may contribute to dictate the cytokine profile in T helper type 1 responses induced by DCs.
Introduction
The Toll-like receptor (TLR)–mediated recognition of microbial component by dendritic cells (DCs), the most potent professional antigen-presenting cells, is an event central to the activation of innate and adaptive immune responses.1,2 TLRs recognize pathogen-associated molecular patterns that are common to many organisms. Although individual TLR agonists are unique in their response profile, they apparently use common activation pathways. The hallmark of TLR signaling is the induction of various cytokines and chemokines. Several in vitro and in vivo studies have demonstrated that TLR stimulation induces different, but overlapping T helper (Th)1-Th2 cytokine/chemokine profiles suggestive of a complex interplay of stimulatory and inhibitory signals. It is now thought that, to induce an effective immune response, microorganisms must stimulate complex sets of patter-recognition receptors (PRRs), both within and outside of the TLR family. Growing evidence suggests that the fate of T-cell responses depends strongly on the type of PRR triggered and the timing of PRR signaling.3 The combined activation of these different receptors can result in complementary, synergistic, or antagonistic effects that modulate innate and adaptive immunity. In the setting of infection, it seems now likely that multiple TLR pathways are activated by different microorganism components, and that interplay between TLR signaling pathways may have important effects on host inflammatory responses and on its outcome. Thus, a complete understanding of the role of TLRs in host response to pathogens requires decoding of these multiple receptor interactions.4
CC chemokine ligand 2 (CCL2) was originally described as a potent chemoattractant for monocytes. This chemokine is produced by a variety of cell types, including endothelial cells and cells of the monocytic lineage in response to different signals, such as cytokines, lipopolysaccharide (LPS), platelet-derived growth factor, pathogens, and oxidized low-density lipoprotein.5-9 Many activities have been subsequently assigned to this protein, including chemotaxis of mononuclear phagocytes, basophils, T lymphocytes and natural killer cells,10 modulation of tumor growth in animal models,11 and modulation of HIV-1 infection.12,13 It is now broadly documented that CCL2 plays a critical role in inflammatory diseases, and enhanced levels of this chemokine are commonly observed in some autoimmune disorders.14 Furthermore, CCL2 can influence both innate and adaptive immunity, by affecting monocyte and DC biology and functions15 and influencing Th cell differentiation, respectively.16,17
We report in this study that simultaneous ligation of TLR4 and TLR8, although leading to synergistic activation of interleukin (IL)-12 and other cytokine secretion, concomitantly results in a marked reduction of CCL2 production, suggesting that a selective down-modulation of this chemokine can play a critical role for an optimal Th type 1 polarization.
These results add further evidence for a pivotal role of CCL2 in immune regulation and may provide the rationale for the design of novel therapeutic strategies aimed at manipulating DC-mediated immune responses.
Methods
Cell separation and culture
Monocytes were isolated from peripheral blood mononuclear cells obtained from healthy donor buffy coats by immunomagnetic selection using CD14 microbeads (MACS monocyte isolation kit from Miltenyi Biotec), according to the manufacturer's instructions. This procedure yields at least a 98% pure population of monocytes, as assessed by fluorescence-activated cell sorter analysis of lineage-specific surface markers (CD1a, CD14, CD3, CD19, CD56). To obtain immature monocyte-derived DCs (MD-DCs), monocytes were cultured at 106 cells/mL in RPMI 1640 medium (Life Technologies) containing 10% fetal bovine serum in the presence of granulocyte-macrophage colony-stimulating factor (50 ng/mL) and IL-4 (500 U/mL). Cytokines were added to the cultures every 3 days. Both cytokines were provided by Schering-Plough. On day 5, MD-DCs were stimulated with LPS (100 ng/mL), resiquimod (R848) (2 μg/mL), imiquimod (R837) (2 μg/mL), polyinosinic:polycytidylic acid [p(I:C)] (20 μg/mL), or their combination.
Reagents
All culture reagents were purchased from HyClone as endotoxin-free lots and further assessed by the Limulus amebocyte assay (BioWhittaker). Unpurified LPS from Escherichia coli (serotype 026:B6), p(I:C), and N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK) were purchased from Sigma-Aldrich; imiquimod (R837) and ultrapure LPS from E coli (serotype EH100) were purchased from InvivoGen and from Alexis Biochemicals, respectively, whereas resiquimod (R848) was a gift from Philippe Neuner (Istituto Ricerche di Biologia Moleculaire). Unpurified LPS was used throughout the study, unless differently indicated. To neutralize type I interferons (IFNs), the following antibodies were used: anti-IFNAR1 monoclonal antibody (mAb) 64G12 (20 μg/mL)18 and sheep polyclonal anti-IFN-β antibody (PBL Biomedical Laboratories), at the concentration of 200 neutralizing U/mL. Human recombinant CCL2 was purchased from PeproTech, whereas the anti–IL-10 anti–tumor necrosis factor (TNF)-α and control IgG2b antibodies were purchased from R&D Systems.
Cytokine and chemokine determination
Cytokine and chemokine levels in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems for IL-12 p70, CCL1, CCL2, and CCL4, and from Pierce Endogen for IFN-γ and IL-10. Type I IFNs secreted in culture supernatants were measured by using a cytopathic effect reduction assay.19
Real-time reverse transcription-polymerase chain reaction analysis
Total RNA isolated with the Qiagen RNeasy mini kit was treated with DNase I (Qiagen) and retrotranscribed into cDNA by using polyd(N)6 (GE Healthcare). Real-time polymerase chain reaction (PCR) was performed on an ABI-Prism 7700 PCR cycler (Applied Biosystems) using the TaqMan universal master mix (Applied Biosystems), according to the manufacturer's instructions. Validated PCR primers and TaqMan minor groove binder probe (6-carboxyfluorescein labeled) for CCL2, IL-12, and the human β-actin as endogenous control were used (Applied Biosystems). Relative quantification was performed by using the comparative cycle threshold (Ct) method.20
Detection of nuclear factor-κB activation
Nuclear cell extracts were prepared from TLR agonist-treated and untreated DCs by using a nuclear extract kit (Active Motif), and the activity of nuclear factor (NF)-κB p65 was measured with a NF-κB transcription factor assay kit (TransAM; Active Motif), following the manufacturer's instructions. In brief, 5 μg of nuclear cell extract was added to each well coated with consensus-binding site oligonucleotides of NF-κB p65 in the presence or in the absence of competitive or mutated oligonucleotides. A primary antibody specific for an epitope on the bound and active form of the transcription factor was then added, followed by subsequent incubation with a secondary antibody conjugated to horseradish peroxidase.
MicroRNA profiling and data analysis
For microRNA (miR) profiling, total RNA was extracted by TRIzol, according to manufacturer's instructions (Invitrogen). RNA (5 μg) was used for miR microarray profiling (LC-Sciences) that included 4 replicas of 723 mature human miR probes (miRBase 10.1, http://microrna.sanger.ac.uk/sequences). Background was subtracted from raw data, and the signal value for each probe was considered to be detectable if it met 3 conditions, as follows: (1) signal intensity more than 3 times the background standard deviation; (2) spot coefficient of variation (CV) < 0.5, in which CV = standard deviation/signal intensity; and (3) miR listed as detectable if at least 50% of its repeating probes were above the detection level. Detectable signals were normalized to remove system-related variations using quantile normalization (affy package)21 with the open-source software R (Bioconductor [http://www.bioconductor.org/]).22 Normalized data were imported and analyzed in GeneSpring (version 7.2; Agilent Technologies). Differentially expressed miR with P values less than .05 were hierarchically clustered using the average linkage algorithm and the standard correlation as similarity measure.
For miR validation, real-time PCR was performed starting from 5 ng of total RNA coming from the same samples used for miR profiling. Specific stem-loop primers were used for the validation of mature miR.23 The endogenous control let-7a was used for normalization. All microarray data have been deposited with ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae/) under accession number E-MEXP-2183.
Sorting and priming of naive T cells
Naive CD4+CD45RA+ T cells were isolated from peripheral blood mononuclear cells with anti-CD4 magnetic beads (Miltenyi Biotec) to more than 95% purity as CD45RA+CD25− after being stained with fluorescein isothiocyanate-conjugated anti-CD25, phycoerythrin-conjugated anti-CD45RA antibody. MD-DCs were stimulated for 18 hours with TLR agonists, washed, and plated with allogeneic naive T cells at a ratio of 1:10 in 24-well plates. On day 5, proliferating cell populations were expanded with IL-2 (10 IU/mL), and after 7 days, cells and supernatants were collected for fluorescence-activated cell sorter analysis and cytokine measurement, respectively.
Intracellular cytokine staining
For intracellular cytokine detection, T cells were stimulated for 5 hours with phorbol 12-myristate 13-acetate plus ionomycin. GolgiStop (BD Biosciences) was added for the final 3 hours of culture. Then cells were fixed and made permeable with BD Cytofix/Cytoperm kit (BD Biosciences), following the manufacturer's instructions, and stained with fluorescein isothiocyanate-labeled mAb to IFN-γ and phycoerythrin-labeled mAb to IL-4 (BD Biosciences). Cells were analyzed on a FACSCalibur cytometer (BD Biosciences).
Statistical analysis
Statistical comparison between various groups was performed by the one-way analysis of variance (ANOVA) with either least significant difference or Bonferroni post hoc tests, and by the paired Student t test for independent samples, as appropriate, using the SPSS software (version 12.0.2) for statistical analysis. Comparisons were made between means from several experiments. Differences were considered significant when P values were less than .05. Statistical significance is indicated for P values less than .05 (*) and P values less than .005 (**).
Results
CCL2 production, induced by engagement of single TLRs, is inhibited by selected agonist combinations in MD-DCs
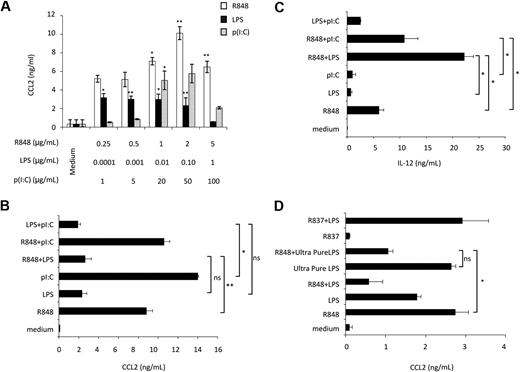
Initial experiments were performed to evaluate the effect of ligands, engaging MD-DC–expressed TLRs, on CCL2 production. p(I:C), LPS, and R848, which trigger TLR3, TLR4, and TLR8, respectively, were used as agonists.24 As shown in Figure 1A, p(I:C) and R848 stimulated CCL2 secretion in a concentration-dependent manner. Human DC stimulation with selected TLR agonist combinations synergistically induces the expression of a variety of soluble factors.25,26 We thus sought to determine whether distinct TLRs may also act in synergy in the induction of CCL2. As shown in Figure 1B, the simultaneous addition of LPS and R848 did not result in any enhancement of CCL2 secretion, but, rather unexpectedly, led to a marked reduction in the production of this chemokine with respect to the addition of R848 alone. A similar inhibitory effect was also observed upon simultaneous addition of LPS and p(I:C). Conversely, treatment of MD-DCs with the agonist combination R848 and p(I:C) did not result in any significant change in CCL2 production. In keeping with previous observations,25 the simultaneous addition of R848 and LPS or p(I:C) markedly up-modulated IL-12 secretion, whereas the combination of LPS and p(I:C) did not significantly affect IL-12 production with respect to the addition of a single agonist (Figure 1C). Because R848 has been reported to trigger both TLR7 and TLR8 and most of the commonly used LPS can also engage TLR2,27 experiments were performed to define the specificity of TLR4 and TLR8 triggering in our experimental conditions. As shown in Figure 1D, treatment of MD-DCs with R837, a specific TLR7 agonist,28 did not induce any CCL2 secretion and did not show any effect when combined with LPS, whereas ultrapure LPS, triggering specifically TLR4, but not TLR2, induced CCL2 production and interfered with that triggered by R848 to a similar extent than unpurified LPS.
Effect of single or combined treatment with TLR3, TLR4, and TLR8 agonists on the secretion of CCL2 in MD-DCs. (A) MD-DCs at day 5 of culture were exposed to different concentrations of p(I:C), LPS, and R848. After 18 hours, supernatants were collected and CCL2 content was measured by ELISA. Data are expressed as mean ± SD of culture duplicates and are representative of 5 independent experiments. P values were calculated by ANOVA comparing results from TLR ligand-stimulated versus unstimulated cells. (B-C) MD-DCs were treated with p(I:C) (20 μg/mL), LPS (100 ng/mL), or R848 (2 μg/mL), either alone or in combination. Eighteen hours later, supernatants were collected for CCL2 (B) and IL-12p70 (C) determination. Results are presented as means ± SD of duplicate wells from one representative of 6 independent experiments. P values were calculated by ANOVA among the single and combined TLR ligand-stimulated cells. (D) MD-DCs were treated with unpurified LPS (100 ng/mL), ultrapure LPS (100 ng/mL), R837 (2 μg/mL), or R848 (2 μg/mL), either alone or in combination. Eighteen hours later, supernatants were collected for CCL2 determination. Results are presented as means ± SD of duplicated wells from one representative of 5 independent experiments. P values were calculated by ANOVA and indicate significant reduction of CCL2 content in cultures simultaneously treated with ultrapure LPS and R848 versus cultures treated with R848 alone. Conversely, no significant modulation in CCL2 content was observed in cultures treated with R837 alone with respect to control cultures.
Effect of single or combined treatment with TLR3, TLR4, and TLR8 agonists on the secretion of CCL2 in MD-DCs. (A) MD-DCs at day 5 of culture were exposed to different concentrations of p(I:C), LPS, and R848. After 18 hours, supernatants were collected and CCL2 content was measured by ELISA. Data are expressed as mean ± SD of culture duplicates and are representative of 5 independent experiments. P values were calculated by ANOVA comparing results from TLR ligand-stimulated versus unstimulated cells. (B-C) MD-DCs were treated with p(I:C) (20 μg/mL), LPS (100 ng/mL), or R848 (2 μg/mL), either alone or in combination. Eighteen hours later, supernatants were collected for CCL2 (B) and IL-12p70 (C) determination. Results are presented as means ± SD of duplicate wells from one representative of 6 independent experiments. P values were calculated by ANOVA among the single and combined TLR ligand-stimulated cells. (D) MD-DCs were treated with unpurified LPS (100 ng/mL), ultrapure LPS (100 ng/mL), R837 (2 μg/mL), or R848 (2 μg/mL), either alone or in combination. Eighteen hours later, supernatants were collected for CCL2 determination. Results are presented as means ± SD of duplicated wells from one representative of 5 independent experiments. P values were calculated by ANOVA and indicate significant reduction of CCL2 content in cultures simultaneously treated with ultrapure LPS and R848 versus cultures treated with R848 alone. Conversely, no significant modulation in CCL2 content was observed in cultures treated with R837 alone with respect to control cultures.
Overall, these results indicate that distinct TLRs may either cooperate or act independently, and that when cooperation occurs it may have different outcomes, either synergy or inhibition, depending on both the selected agonist combination and the cytokine/chemokine. Furthermore, although LPS per se stimulates CCL2 secretion, it appears to exert a dominant-negative effect on the expression of this chemokine when concomitantly administered with TLR3 and TLR8 agonists.
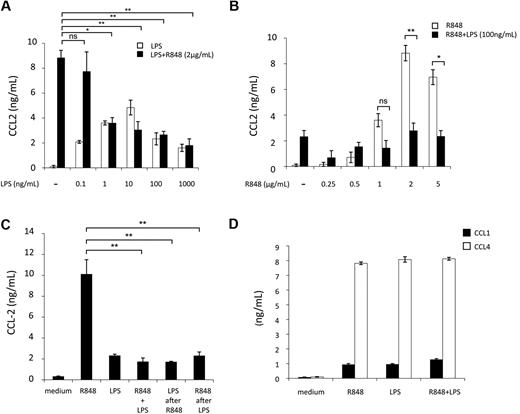
To further investigate this inhibitory effect, we focused on the LPS and R848 agonist combination. First, we analyzed the concentration requirements for triggering CCL2 inhibition by challenging MD-DCs with a range of LPS and R848 concentrations. As shown in Figure 2A, the inhibition of R848-induced (2 μg/mL) CCL2 secretion was already observed at the LPS concentration of 1 ng/mL and slightly increased at higher LPS concentrations. When the fixed concentration of LPS (100 ng/mL) was used in combination with different R848 concentrations, the inhibitory effect was observed at the R848 concentration of 1 μg/mL and further increased at higher R848 concentrations (Figure 2B).
Concentration, temporal requirements, and specificity of TLR4 and TLR8 agonist combination for CCL2 inhibition. MD-DCs were treated with a range of concentrations of either LPS or R848. (A) Cultures received fixed amount of R848 (2 μg/mL) and variable concentrations of LPS ranging from 0.1 up to 1000 ng/mL. Data are expressed as mean ± SD of culture duplicates and are representative of 6 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from cultures simultaneously treated with different amounts of LPS and a fixed concentration of R848 with respect to cultures treated with R848 alone. (B) Cultures received a fixed amount of LPS (100 ng/mL) and variable concentrations of R848 ranging from 0.25 up to 5 μg/mL. Eighteen hours later, supernatants were collected for CCL2 content determination. Data are expressed as mean ± SD of culture duplicates and are representative of 6 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from cultures treated with different concentrations of R848 in the presence or in the absence of a fixed amount of LPS. (C) MD-DCs were stimulated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination. Stimuli were added either simultaneously at time 0 or sequentially, with LPS being added 3 hours after R848 or vice versa, R848 3 hours after LPS. CCL2 secretion was measured 18 hours later. Data are expressed as mean ± SD of culture duplicates and are representative of 4 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from cultures simultaneously treated with LPS and R848 with respect to cultures treated with R848 alone.(D) MD-DCs were treated with LPS (100 ng/mL) and R848 (2 μg/mL) alone or in combination. Eighteen hours later, supernatants were collected for CCL1 and CCL4 content determination. Data are expressed as mean ± SD of culture duplicates and are representative of 3 independent experiments. P values were calculated by ANOVA and indicated no significant modulation in CCL1 or CCL4 content in cultures simultaneously treated with LPS and R848 with respect to cultures treated with a single agonist.
Concentration, temporal requirements, and specificity of TLR4 and TLR8 agonist combination for CCL2 inhibition. MD-DCs were treated with a range of concentrations of either LPS or R848. (A) Cultures received fixed amount of R848 (2 μg/mL) and variable concentrations of LPS ranging from 0.1 up to 1000 ng/mL. Data are expressed as mean ± SD of culture duplicates and are representative of 6 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from cultures simultaneously treated with different amounts of LPS and a fixed concentration of R848 with respect to cultures treated with R848 alone. (B) Cultures received a fixed amount of LPS (100 ng/mL) and variable concentrations of R848 ranging from 0.25 up to 5 μg/mL. Eighteen hours later, supernatants were collected for CCL2 content determination. Data are expressed as mean ± SD of culture duplicates and are representative of 6 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from cultures treated with different concentrations of R848 in the presence or in the absence of a fixed amount of LPS. (C) MD-DCs were stimulated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination. Stimuli were added either simultaneously at time 0 or sequentially, with LPS being added 3 hours after R848 or vice versa, R848 3 hours after LPS. CCL2 secretion was measured 18 hours later. Data are expressed as mean ± SD of culture duplicates and are representative of 4 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from cultures simultaneously treated with LPS and R848 with respect to cultures treated with R848 alone.(D) MD-DCs were treated with LPS (100 ng/mL) and R848 (2 μg/mL) alone or in combination. Eighteen hours later, supernatants were collected for CCL1 and CCL4 content determination. Data are expressed as mean ± SD of culture duplicates and are representative of 3 independent experiments. P values were calculated by ANOVA and indicated no significant modulation in CCL1 or CCL4 content in cultures simultaneously treated with LPS and R848 with respect to cultures treated with a single agonist.
TLRs are present in different cellular compartments, suggesting that they may be triggered with different kinetics. Furthermore, after initial TLR stimulation, DCs become refractory to subsequent stimulation by the same or other TLR agonists. Thus, we investigated whether the timing and order of agonist addition would affect the extent of inhibition of CCL2 secretion. As shown in Figure 2C, we found no significant changes in the extent of CCL2 inhibition when LPS and R848 were added within a 3-hour window and regardless of the addition order. To define whether the inhibitory effect on CCL2 secretion was specific for this chemokine or might be extended to other CC chemokines, the capacity of LPS and R848 combination to modulate CCL1 and CCL4 secretion was also investigated. As shown in Figure 2D, the simultaneous engagement of TLR4 and TLR8 did not result in any significant modulation of CCL1 and CCL4 secretion with respect to the single agonists.
Synergistic induction of IFN-β, TNF-α, or IL-10 by combined TLR engagement does not contribute to CCL2 secretion inhibition
Type I IFN induction has been demonstrated to play an important role in the cascade of gene expression after TLR signaling.29,30 These cytokines are rapidly produced after induction of MD-DC maturation by engagement of TLRs,31,32 and act as CCL2 inducers in human macrophages.33 Thus, we next evaluated whether type I IFN had some role in the regulation of CCL2 production triggered in MD-DCs by TLR engagement. Because type I IFN represents a family of proteins including IFN-β and at least 13 different subtypes of IFN-α sharing a common type I IFN receptor, a mAb directed against this receptor was added to MD-DC cultures before TLR agonist stimulation. As shown in Figure 3A, blocking type I IFN activity markedly reduced the secretion of CCL2 induced by LPS or R848, although it did not significantly reduce the already low level of CCL2 detected after combined TLR4 and TLR8 stimulation. These results indicate that CCL2 secretion is not a direct response to TLR engagement, but it is subsequently triggered as a consequence of type I IFN secretion, directly and rapidly released upon TLR triggering. Thus, we next evaluated whether a reduced type I IFN production, eventually occurring upon combined TLR engagement, could account for the reduced CCL2 secretion. As shown in Figure 3B, little amounts of type I IFN were detected in MD-DC culture supernatants after treatment with LPS or R848 alone, whereas a higher production was observed after stimulation with the agonist combination. In keeping with previous results,31,32 IFN-β was the major IFN component released because the addition of a specific anti-IFN-β antibody almost completely neutralized the antiviral activity detected in the MD-DC culture supernatants.
Effect of cytokines, synergistically induced by combined TLR engagement, on CCL2 induction and regulation. (A) MD-DCs were treated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination, in the presence or in the absence of a neutralizing mAb to type I IFN receptor (20 μg/mL) or control antibody. Eighteen hours later, supernatants were collected and tested for CCL2 content. Results are presented as means ± SD of duplicate wells from 1 representative of 6 independent experiments. P values were calculated by Student t test, and statistical significance is indicated comparing results from cultures treated with LPS or R848 or their combination in the presence of type I IFN receptor (IFN-R)–blocking mAb with those achieved in the absence of the mAb. (B) MD-DCs were stimulated as described in (A). Eighteen hours later, supernatants were collected and the biologically active type I IFN content in the culture medium was titered as described in “Cytokine and chemokine determination.” The content of IFN-β was determined by the addition of a specific anti-IFN-β antibody in the titration assay. A representative experiment of 3 performed is shown. (C-D) MD-DCs were activated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination, in the presence or in the absence of a mAb to TNF-α (100 ng/mL) and its control antibody (C) or a mAb to IL-10 (500 ng/mL) and its control antibody (D). Eighteen hours later, supernatants were collected and tested for CCL2 content. Results are presented as means ± SD of duplicate wells from 1 representative of 3 independent experiments. P values were calculated by paired Student t test and indicate no significant changes in CCL2 content in cultures treated with LPS or R848 or their combination in the presence or absence of mAb to TNF-α or IL-10, respectively.
Effect of cytokines, synergistically induced by combined TLR engagement, on CCL2 induction and regulation. (A) MD-DCs were treated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination, in the presence or in the absence of a neutralizing mAb to type I IFN receptor (20 μg/mL) or control antibody. Eighteen hours later, supernatants were collected and tested for CCL2 content. Results are presented as means ± SD of duplicate wells from 1 representative of 6 independent experiments. P values were calculated by Student t test, and statistical significance is indicated comparing results from cultures treated with LPS or R848 or their combination in the presence of type I IFN receptor (IFN-R)–blocking mAb with those achieved in the absence of the mAb. (B) MD-DCs were stimulated as described in (A). Eighteen hours later, supernatants were collected and the biologically active type I IFN content in the culture medium was titered as described in “Cytokine and chemokine determination.” The content of IFN-β was determined by the addition of a specific anti-IFN-β antibody in the titration assay. A representative experiment of 3 performed is shown. (C-D) MD-DCs were activated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination, in the presence or in the absence of a mAb to TNF-α (100 ng/mL) and its control antibody (C) or a mAb to IL-10 (500 ng/mL) and its control antibody (D). Eighteen hours later, supernatants were collected and tested for CCL2 content. Results are presented as means ± SD of duplicate wells from 1 representative of 3 independent experiments. P values were calculated by paired Student t test and indicate no significant changes in CCL2 content in cultures treated with LPS or R848 or their combination in the presence or absence of mAb to TNF-α or IL-10, respectively.
To gain further insights into the mechanisms underlying TLR interference, we investigated the role of selected cytokines (ie, TNF-α and IL-10), synergistically induced in response to R848 and LPS25 and previously reported to be involved in the modulation of CCL2 expression. As shown in Figure 3C, the addition of neutralizing antibody to TNF-α did not exert any effect on the production of CCL2 induced by R848, LPS, or their combination, thus excluding a role for this cytokine in both CCL2 induction and negative regulation. Likewise, as shown in Figure 3D, pretreatment with neutralizing antibodies to IL-10 neither enhances CCL2 secretion induced by the single agonists nor rescues CCL2 secretion inhibited by agonist combination.
Triggering of MD-DCs with TLR4 and TLR8 agonist combination strongly reduces CCL2 mRNA accumulation and impairs NF-κB p65 activation
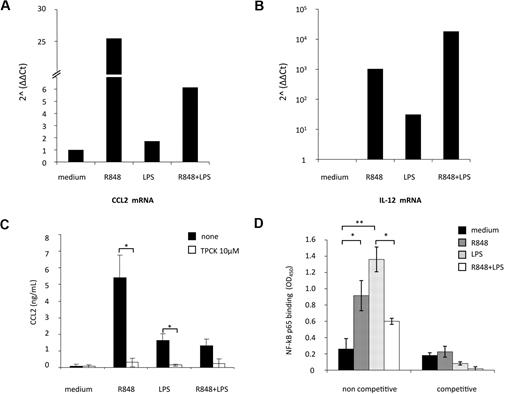
Real-time PCR for CCL2 transcript was then performed in MD-DCs exposed to the single agonists or simultaneously treated with LPS and R848. As shown in Figure 4A, CCL2 mRNA levels were modestly up-modulated by LPS (1.7-fold increase), whereas a marked enhancement in the accumulation of this transcript was observed upon R848 treatment (26-fold increase). Simultaneous stimulation of MD-DCs with LPS and R848 led to a marked reduction (4-fold decrease) in CCL2 transcript accumulation with respect to the levels observed after treatment with R848 alone. In contrast, IL-12p40 mRNA expression, used as a control, was more sustained after combined TLR stimulation, with respect to the single treatment (Figure 4B), as previously reported.25
TLR4 and TLR8 agonist combination markedly reduces CCL2 mRNA accumulation: role of NF-κB signaling pathway. (A-B) MD-DCs were treated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination. After 6 hours, total RNA was extracted and retrotranscribed. Real-time PCR was performed using primers specific for human CCL2 (A) and IL-12p40 (B) mRNA on cDNA normalized with β-actin. One representative experiment of 3 performed is shown. Results, analyzed by the relative quantification method (2−ΔΔCt method), are presented as fold increase/reduction of CCL2 and IL-12p40 gene expression. (C) MD-DCs were treated with of 10 μM TPCK or left untreated. At the concentration used, this inhibitor did not affect cell viability (data not shown). After 30 minutes, some cultures were exposed to TLR ligands, as described in the legend to Figure 2. After 18 hours of culture, supernatants were harvested and frozen for CCL2 determination. Data are expressed as mean ± SD of duplicate wells and are representative of 4 independent experiments. P values were calculated by Student t test, and statistical significance is indicated comparing results from TPCK-treated cells versus untreated cells. (D) NF-κB activation was measured in nuclear extracts (5 μg) after a 2-hour stimulation with TLR ligands by a transcription factor ELISA. Data are expressed as mean ± SD of duplicate wells and are representative of 3 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from MD-DCs treated with single agonists versus control cells and from MD-DCs treated with a combination of the agonists versus the single treatments.
TLR4 and TLR8 agonist combination markedly reduces CCL2 mRNA accumulation: role of NF-κB signaling pathway. (A-B) MD-DCs were treated with LPS (100 ng/mL) or R848 (2 μg/mL) alone or in combination. After 6 hours, total RNA was extracted and retrotranscribed. Real-time PCR was performed using primers specific for human CCL2 (A) and IL-12p40 (B) mRNA on cDNA normalized with β-actin. One representative experiment of 3 performed is shown. Results, analyzed by the relative quantification method (2−ΔΔCt method), are presented as fold increase/reduction of CCL2 and IL-12p40 gene expression. (C) MD-DCs were treated with of 10 μM TPCK or left untreated. At the concentration used, this inhibitor did not affect cell viability (data not shown). After 30 minutes, some cultures were exposed to TLR ligands, as described in the legend to Figure 2. After 18 hours of culture, supernatants were harvested and frozen for CCL2 determination. Data are expressed as mean ± SD of duplicate wells and are representative of 4 independent experiments. P values were calculated by Student t test, and statistical significance is indicated comparing results from TPCK-treated cells versus untreated cells. (D) NF-κB activation was measured in nuclear extracts (5 μg) after a 2-hour stimulation with TLR ligands by a transcription factor ELISA. Data are expressed as mean ± SD of duplicate wells and are representative of 3 independent experiments. P values were calculated by ANOVA, and statistical significance is indicated comparing results from MD-DCs treated with single agonists versus control cells and from MD-DCs treated with a combination of the agonists versus the single treatments.
CCL2 expression strongly depends on NF-κB activation in various cell types, and binding of p65/p65 and c-Rel/p65 dimers to NF-κB sites of the CCL2 promoter has been reported to enhance the transcription of this gene.34,35 To evaluate whether NF-κB activation was involved in TLR4 and TLR8 agonist-mediated induction of CCL2, we initially tested the effect of the protease inhibitor TPCK, which blocks NF-κB activation by preventing inhibitory NF-κB proteolytic degradation, on the secretion of this chemokine. As shown in Figure 4C, TPCK completely abrogated CCL2 secretion induced by the single agonists, whereas it did not further reduce the already low level of CCL2 secretion observed in the presence of agonist combination.
We then evaluated whether stimulation of MD-DCs with agonist combination could somehow modify the NF-κB activation profile. To this aim, DNA-binding capacity of different NF-κB subunits was analyzed. As shown in Figure 4D, p65 binding to NF-κB site was markedly increased after stimulation with LPS or R848. However, when LPS and R848 were administered together, p65-binding activity was not synergistically up-modulated, but rather decreased with respect to the single agonists. Conversely, the binding capacity of other NF-κB subunits (p50, p52, c-Rel, and RelB), modulated at different extents by single agonists, did not vary upon combined agonist stimulation (data not shown).
TLR4 and TLR8 engagement differentially affects miR expression profile in MD-DCs
miR binding to complementary sequences, usually located in the 3′-untranslated regions of mRNA, can regulate the expression of large groups of genes by a variety of mechanisms.36 To understand whether these regulators of gene expression could somehow contribute to the fine tuning of CCL2 expression in MD-DCs, we performed a large-scale miR expression profiling analysis of 723 human mature miR on MD-DCs, either untreated or stimulated with LPS, R848, or their combination for 8 hours. As shown in Table 1, after normalization and filtering, 70 of 93 detectable mature miR were found to be consistently expressed (with average signal values > 500) in control MD-DCs, although with some variability in the extent of expression among donors (n = 3). The constitutively expressed miR in MD-DCs were grouped in 3 different categories, on the basis of their data distribution percentiles. Specifically, 18 miR were found to be highly expressed, 34 displayed an intermediate expression, whereas 18 showed low expression. Notably, some of the miR detected in MD-DCs have been previously characterized as regulators of immune cell development and function (miR-125, miR-132, miR-146, and miR-155),37 associated with hematopoietic cell differentiation (miR-106a/363 cluster, miR-17/92 cluster, miR-223, miR-424), or reported to exhibit an increased expression in differentiated cells and associated with the maintenance of the differentiated state (miR let-7/98 family).38
miR expression pattern in MD-DCs
| High . | Intermediate . | Low . | |||
|---|---|---|---|---|---|
| miR . | Mean ± SD . | miR . | Mean ± SD . | miR . | Mean ± SD . |
| miR-23a | 27316 ± 3574 | miR-923 | 11578 ± 5702 | hsa-miR-92b | 2328 ± 1400 |
| miR-23b | 24971 ± 3559 | miR-29a | 9851 ± 2720 | hsa-miR-181a | 2103 ± 936 |
| miR-223 | 22762 ± 3700 | let-7b | 9193 ± 2766 | hsa-miR-422a | 2031 ± 1271 |
| miR-342-3p | 20967 ± 5289 | miR-27a | 8995 ± 2937 | hsa-miR-125b | 1981 ± 687 |
| let-7a | 20940 ± 2710 | miR-638 | 8122 ± 4298 | hsa-miR-30a | 1891 ± 494 |
| miR-146b-5p | 20464 ± 2488 | hsa-miR-30b | 8099 ± 1687 | hsa-miR-93 | 1755 ± 840 |
| miR-16 | 20438 ± 3200 | hsa-miR-92a | 8019 ± 3837 | hsa-miR-106b | 1509 ± 434 |
| miR-26a | 19895 ± 1525 | hsa-miR-320 | 7786 ± 1306 | hsa-miR-28-5p | 1489 ± 553 |
| let-7f | 19628 ± 2321 | hsa-miR-30c | 7617 ± 1317 | hsa-miR-20b | 1339 ± 545 |
| miR-191 | 18215 ± 3069 | hsa-miR-15b | 7521 ± 1645 | hsa-miR-148b | 1312 ± 448 |
| let-7g | 17256 ± 1161 | hsa-miR-15a | 7106 ± 1064 | hsa-miR-151-5p | 1302 ± 150 |
| let-7d | 16510 ± 2162 | hsa-let-7e | 6649 ± 3590 | hsa-miR-374b | 1143 ± 339 |
| miR-103 | 15565 ± 3255 | hsa-miR-221 | 6578 ± 1952 | hsa-miR-532-5p | 1131 ± 437 |
| miR-107 | 14810 ± 3231 | hsa-miR-425 | 5177 ± 2858 | hsa-miR-30e | 1076 ± 249 |
| let-7c | 14527 ± 1439 | hsa-miR-22 | 5096 ± 2848 | hsa-miR-128 | 1062 ± 315 |
| miR-26b | 13323 ± 3124 | hsa-miR-146a | 4830 ± 1566 | hsa-miR-660 | 841 ± 312 |
| let-7i | 12984 ± 2023 | hsa-miR-17 | 4582 ± 1459 | hsa-miR-342-5p | 795 ± 192 |
| miR-24 | 12363 ± 2808 | hsa-miR-423-5p | 4337 ± 799 | hsa-miR-155 | 711 ± 202 |
| hsa-miR-25 | 4255 ± 685 | ||||
| hsa-miR-20a | 4254 ± 924 | ||||
| hsa-miR-106a | 4204 ± 1361 | ||||
| hsa-miR-132 | 3968 ± 1392 | ||||
| hsa-miR-19b | 3943 ± 2315 | ||||
| hsa-miR-222 | 3862 ± 772 | ||||
| hsa-miR-424 | 3387 ± 1365 | ||||
| hsa-miR-185 | 3297 ± 497 | ||||
| hsa-miR-378 | 3287 ± 1609 | ||||
| hsa-miR-361-5p | 3039 ± 576 | ||||
| hsa-miR-29c | 2953 ± 796 | ||||
| hsa-miR-27b | 2943 ± 1018 | ||||
| hsa-miR-140-3p | 2942 ± 1091 | ||||
| hsa-miR-30d | 2740 ± 466 | ||||
| hsa-miR-142-5p | 2533 ± 1275 | ||||
| hsa-miR-663 | 2380 ± 1816 | ||||
| High . | Intermediate . | Low . | |||
|---|---|---|---|---|---|
| miR . | Mean ± SD . | miR . | Mean ± SD . | miR . | Mean ± SD . |
| miR-23a | 27316 ± 3574 | miR-923 | 11578 ± 5702 | hsa-miR-92b | 2328 ± 1400 |
| miR-23b | 24971 ± 3559 | miR-29a | 9851 ± 2720 | hsa-miR-181a | 2103 ± 936 |
| miR-223 | 22762 ± 3700 | let-7b | 9193 ± 2766 | hsa-miR-422a | 2031 ± 1271 |
| miR-342-3p | 20967 ± 5289 | miR-27a | 8995 ± 2937 | hsa-miR-125b | 1981 ± 687 |
| let-7a | 20940 ± 2710 | miR-638 | 8122 ± 4298 | hsa-miR-30a | 1891 ± 494 |
| miR-146b-5p | 20464 ± 2488 | hsa-miR-30b | 8099 ± 1687 | hsa-miR-93 | 1755 ± 840 |
| miR-16 | 20438 ± 3200 | hsa-miR-92a | 8019 ± 3837 | hsa-miR-106b | 1509 ± 434 |
| miR-26a | 19895 ± 1525 | hsa-miR-320 | 7786 ± 1306 | hsa-miR-28-5p | 1489 ± 553 |
| let-7f | 19628 ± 2321 | hsa-miR-30c | 7617 ± 1317 | hsa-miR-20b | 1339 ± 545 |
| miR-191 | 18215 ± 3069 | hsa-miR-15b | 7521 ± 1645 | hsa-miR-148b | 1312 ± 448 |
| let-7g | 17256 ± 1161 | hsa-miR-15a | 7106 ± 1064 | hsa-miR-151-5p | 1302 ± 150 |
| let-7d | 16510 ± 2162 | hsa-let-7e | 6649 ± 3590 | hsa-miR-374b | 1143 ± 339 |
| miR-103 | 15565 ± 3255 | hsa-miR-221 | 6578 ± 1952 | hsa-miR-532-5p | 1131 ± 437 |
| miR-107 | 14810 ± 3231 | hsa-miR-425 | 5177 ± 2858 | hsa-miR-30e | 1076 ± 249 |
| let-7c | 14527 ± 1439 | hsa-miR-22 | 5096 ± 2848 | hsa-miR-128 | 1062 ± 315 |
| miR-26b | 13323 ± 3124 | hsa-miR-146a | 4830 ± 1566 | hsa-miR-660 | 841 ± 312 |
| let-7i | 12984 ± 2023 | hsa-miR-17 | 4582 ± 1459 | hsa-miR-342-5p | 795 ± 192 |
| miR-24 | 12363 ± 2808 | hsa-miR-423-5p | 4337 ± 799 | hsa-miR-155 | 711 ± 202 |
| hsa-miR-25 | 4255 ± 685 | ||||
| hsa-miR-20a | 4254 ± 924 | ||||
| hsa-miR-106a | 4204 ± 1361 | ||||
| hsa-miR-132 | 3968 ± 1392 | ||||
| hsa-miR-19b | 3943 ± 2315 | ||||
| hsa-miR-222 | 3862 ± 772 | ||||
| hsa-miR-424 | 3387 ± 1365 | ||||
| hsa-miR-185 | 3297 ± 497 | ||||
| hsa-miR-378 | 3287 ± 1609 | ||||
| hsa-miR-361-5p | 3039 ± 576 | ||||
| hsa-miR-29c | 2953 ± 796 | ||||
| hsa-miR-27b | 2943 ± 1018 | ||||
| hsa-miR-140-3p | 2942 ± 1091 | ||||
| hsa-miR-30d | 2740 ± 466 | ||||
| hsa-miR-142-5p | 2533 ± 1275 | ||||
| hsa-miR-663 | 2380 ± 1816 | ||||
List of mature homosapiens (hsa) miR with high (expression value higher than 75th percentile), intermediate (25th-75th percentile), or low expression (less than 25th percentile) detected upon microarray miR profiling of MD-DCs. Data are the mean of 3 independent experiments ± SD and are listed according to their decreasing expression.
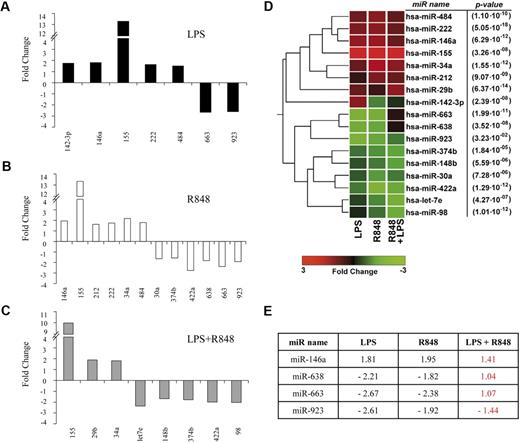
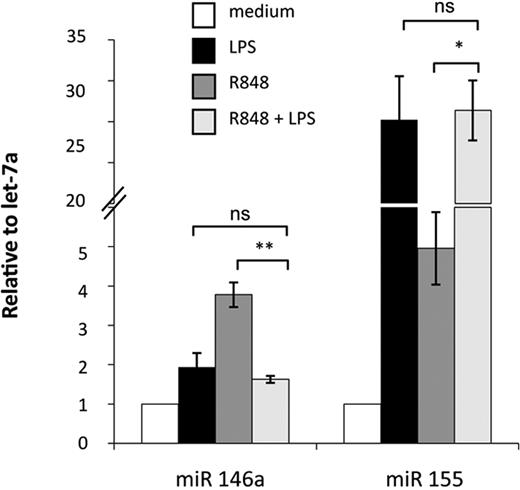
Activation of MD-DCs after treatment with single TLR agonists or their combination resulted in changes in the expression of a panel of miR with respect to control MD-DCs (Figure 5). miR expression data were analyzed for significance by using ANOVA multiple groups test applying the Bonferroni correction. This analysis identified 17 miR that had at least a 1.5-fold difference in expression between the 2 compared parts (LPS-, R848-, or LPS/R848-treated cells versus control cells), along with a P value less than .05 (Figure 5A-C). We then performed an unsupervised, one-way cluster analysis by using standard correlation as similarity measure to hierarchically cluster all the modulated miR, and the corresponding heatmap is shown in Figure 5D. However, comparing the effect of MD-DC–combined treatment with LPS and R848 with that of single agonists, only the differentially expressed miR-146a, miR-638, miR-663, and miR-923 were found to be statistically significant. As shown in Figure 5E, the up-modulation of miR-146a observed upon MD-DCs with LPS or R848 was reverted to the level of nonsignificant modulation (range, <−1.5->1.5) when the agonists were administered together. Similarly, the down-modulation of miR-638, miR-663, and miR-923 observed in cells stimulated with single agonists was reverted to the level of nonsignificant modulation (range, <−1.5->1.5) after combined agonist stimulation. We then used a quantitative real-time reverse transcription-polymerase chain reaction to validate the expression pattern of the above described modulated miR. Specifically, miR-146a and miR-155 were initially chosen to confirm the significance of microarray results. miR-146a was chosen as a representative example of differentially modulated miR in combined agonist-stimulated cells versus single agonists, whereas miR-155 was selected for the extent of its modulation. As shown in Figure 6, the up-regulation of miR-146a was significantly reduced in cells treated with agonist combination with respect to R848-, but not LPS-treated cells. On the contrary, the level of expression of the miR-155 did not significantly change upon stimulation with agonist combination with respect to single treatments, in keeping with the results shown in Figure 5E.
Modulation of miR expression profile by TLR agonists. (A-C) MiR up- or down-modulated at least 1.5-fold in MD-DCs treated for 8 hours with R848 (2 μg/mL), LPS (100 ng/mL), or their combination with respect to control MD-DCs. Fold change (FC) was calculated dividing the normalized signals of treated samples by the control sample. P values, calculated by ANOVA, were less than .05 for all miR. (D) Dendrogram and heatmap of the modulated miR, shown in (A-C), obtained after cluster analysis. Clustered miR are listed on the right along with their P values. (E) Differentially expressed miR in MD-DCs stimulated with agonist combination versus cells treated with single agonists displayed as FC of expression. P values, calculated by ANOVA, were less than .05 for all miR.
Modulation of miR expression profile by TLR agonists. (A-C) MiR up- or down-modulated at least 1.5-fold in MD-DCs treated for 8 hours with R848 (2 μg/mL), LPS (100 ng/mL), or their combination with respect to control MD-DCs. Fold change (FC) was calculated dividing the normalized signals of treated samples by the control sample. P values, calculated by ANOVA, were less than .05 for all miR. (D) Dendrogram and heatmap of the modulated miR, shown in (A-C), obtained after cluster analysis. Clustered miR are listed on the right along with their P values. (E) Differentially expressed miR in MD-DCs stimulated with agonist combination versus cells treated with single agonists displayed as FC of expression. P values, calculated by ANOVA, were less than .05 for all miR.
Real-time PCR validation of miR-146a and miR-155 expression. Relative FC of expression in stimulated samples against untreated controls were calculated using the comparative Ct (2−ΔΔCt) method. Let-7a was chosen as the endogenous control because it displayed more constant expression values among all treatments with respect to U6 (data not shown). PCRs were run in triplicate, and the mean of 4 independent experiments ± SD is shown. P values were calculated by ANOVA, and statistical significance is indicated comparing results from MD-DCs treated with a combination of agonists versus the single treatments.
Real-time PCR validation of miR-146a and miR-155 expression. Relative FC of expression in stimulated samples against untreated controls were calculated using the comparative Ct (2−ΔΔCt) method. Let-7a was chosen as the endogenous control because it displayed more constant expression values among all treatments with respect to U6 (data not shown). PCRs were run in triplicate, and the mean of 4 independent experiments ± SD is shown. P values were calculated by ANOVA, and statistical significance is indicated comparing results from MD-DCs treated with a combination of agonists versus the single treatments.
CCL2 down-regulation favors DC-mediated Th type 1 polarization
The ensemble of results shown above as well as previously published in vitro and in vivo studies suggest that, at least under specific experimental conditions, an inverse correlation between IL-12 and CCL2 expression generally occurs in DC biology, being the increase of one factor often paralleled by the decrease of the other one. Furthermore, it is known that some chemoattractants, including CCL2, can affect IL-12 production by human monocytes and DCs, although these effects, mostly inhibitory, are more dramatic in monocytes/macrophages than in DCs.39,40 Furthermore, it has been reported that MD-DCs generated in the presence of CCL2 exhibit a reduced CD40-dependent IL-12 production.15
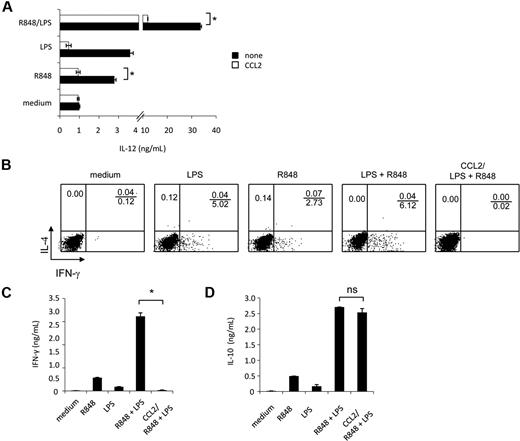
Experiments were then carried out to evaluate whether synergistic IL-12 up-modulation, occurring upon combined TLR agonist treatment, could be negatively affected by reconstitution of CCL2 levels by exogenous addition. As shown in Figure 7A, exogenous addition of CCL2 significantly inhibited IL-12 secretion induced by the synergistic activity of TLR agonist combination as well as by R848 alone. However, the exogenous addition of this chemokine did not significantly affect IL-12 production induced by LPS, although a trend toward reduction was observed.
IL-12 production and polarization of Th1 cells, synergistically induced by combined TLR agonist combination, are inhibited by exogenous CCL2. (A) MD-DCs were seeded and cultured, as described in “Cell separation and culture.” Cells were pretreated for 30 minutes with exogenous CCL2 (50 ng/mL) or left untreated before the addition of LPS (100 ng/mL), R848 (2 μg/mL), or their combination. After 18 hours, supernatants were harvested and frozen for IL-12p70 determination. Data are expressed as mean ± SD of duplicate wells and are representative of 6 independent experiments. The P values were calculated by Student t test, and statistical significance is indicated comparing results from TLR ligand-stimulated cells in the presence versus in the absence of exogenous CCL2. (B-D) MD-DCs were stimulated for 18 hours with LPS, R848, or LPS plus R848 with or without exogenous CCL2 (50 ng/mL), and then were washed and cultured with allogeneic naive CD4+ T cells. (B) Intracellular cytokine staining for IFN-γ and IL-4 in naive CD4+ T cells. After 5 days, cells were incubated for additional 7 days in the presence of IL-2 (10 IU/mL), and then proliferating T cells were tested for their capacity to produce IFN-γ and IL-4 after stimulation with PMA and ionomycin. One representative experiment of 3 is shown. (C) ELISA determination of IFN-γ and (D) IL-10 in culture supernatants of cell populations primed and expanded, as described in (B). Data in (C-D) are expressed as mean ± SD of culture duplicates and are representative of 4 independent experiments. The P values were calculated by ANOVA, and statistical significance is indicated comparing results from TLR ligand-stimulated cells in the presence versus in the absence of exogenous CCL2.
IL-12 production and polarization of Th1 cells, synergistically induced by combined TLR agonist combination, are inhibited by exogenous CCL2. (A) MD-DCs were seeded and cultured, as described in “Cell separation and culture.” Cells were pretreated for 30 minutes with exogenous CCL2 (50 ng/mL) or left untreated before the addition of LPS (100 ng/mL), R848 (2 μg/mL), or their combination. After 18 hours, supernatants were harvested and frozen for IL-12p70 determination. Data are expressed as mean ± SD of duplicate wells and are representative of 6 independent experiments. The P values were calculated by Student t test, and statistical significance is indicated comparing results from TLR ligand-stimulated cells in the presence versus in the absence of exogenous CCL2. (B-D) MD-DCs were stimulated for 18 hours with LPS, R848, or LPS plus R848 with or without exogenous CCL2 (50 ng/mL), and then were washed and cultured with allogeneic naive CD4+ T cells. (B) Intracellular cytokine staining for IFN-γ and IL-4 in naive CD4+ T cells. After 5 days, cells were incubated for additional 7 days in the presence of IL-2 (10 IU/mL), and then proliferating T cells were tested for their capacity to produce IFN-γ and IL-4 after stimulation with PMA and ionomycin. One representative experiment of 3 is shown. (C) ELISA determination of IFN-γ and (D) IL-10 in culture supernatants of cell populations primed and expanded, as described in (B). Data in (C-D) are expressed as mean ± SD of culture duplicates and are representative of 4 independent experiments. The P values were calculated by ANOVA, and statistical significance is indicated comparing results from TLR ligand-stimulated cells in the presence versus in the absence of exogenous CCL2.
Accumulating evidence indicates that CCL2 displays immunoregulatory functions and may be involved in Th subset differentiation. Because selected TLR agonist combinations have been described to synergistically trigger a Th1-type polarizing program in DCs,25 we next evaluated whether the observed down-modulation of CCL2 production was somehow needed to promote this response. To this aim, MD-DCs were stimulated with single agonists or their combination in the presence or in the absence of CCL2, washed, and tested for their capacity to prime naive allogeneic CD4+ T cells. T-cell proliferation was similar in all conditions tested, regardless of the presence of CCL2, and no phenotypic changes were observed in MD-DCs exposed to CCL2 concomitantly to maturation induction (data not shown). As shown in Figure 7B, in keeping with previous observations,25 MD-DCs stimulated by LPS plus R848 induced a much higher Th1 polarization than did MD-DCs stimulated by the single agonists. Interestingly, when CCL2 was added to MD-DCs stimulated by LPS plus R848, IFN-γ production was markedly reduced. However, this impairment in IFN-γ production did not favor the production of IL-4, because this cytokine was not detected under these experimental conditions (Figure 7B). Accordingly, higher levels of IFN-γ were found in the culture medium of DC-T lymphocyte cultures when MD-DCs were stimulated with agonist combination with respect to the single agonists (Figure 7C), whereas IFN-γ production was almost completely abrogated when stimulation with agonist combination occurred in the presence of CCL2. In contrast, IL-10 secretion in the coculture medium was not significantly affected in the presence of exogenous CCL2 (Figure 7D).
Discussion
DCs discriminate different microbial pathogens and induce T-cell responses of appropriate effector phenotype accordingly.41 Microbial recognition and differentiation are mediated in part by PRRs such as TLRs, whereas the development of T-cell effector functions is critically dependent on DC-derived cytokines such as IL-12 and IL-10. Accumulating evidence suggests that the fate of T-cell responses depends strongly on the type of PRR triggered and the timing of PRR signaling.3 However, it is not yet clear to what extent various microbial TLR activators could induce different functional states of DCs that favor different T-cell effector phenotypes. In this regard, several studies have shown that combined TLR ligation synergistically activates DCs, leading to enhanced cytokine production and T-cell responses both in vitro and in vivo.25,26,42 Conversely, little information is currently available on the induction of cross-tolerance between these receptors.43,44
Specifically, we report for the first time that simultaneous engagement of TLR4 and TLR8, whereas synergistically up-modulating IL-12p70 secretion, strongly inhibits CCL2 production. This inhibitory effect is specific of CCL2 because the secretion of several other cytokines/chemokines that we and other groups analyzed25,26,45,46 is either synergistically induced or unaffected under the same experimental conditions. Specificity is also observed in terms of agonist combination, as the inhibitory effect is only observed when TLR4 engagement is combined with TLR3 or TLR8 triggering, suggesting that 2 microbial products need to be present in different cellular compartments, extracellular (TLR4) and endocytic (TLR3 and TLR8), respectively. However, CCL2 down-modulation occurs regardless of the order of agonist addition, thus excluding that timing of TLR stimulation could represent a critical factor for this inhibitory effect.
In this study, we also provide evidence that the observed CCL2 down-modulation is biologically relevant and may play a critical role in the polarization of the immune response. Based on the observation that CCL2 down-modulation often parallels a concomitant up-modulation of IL-12, we reasoned that a regulatory loop balancing the relative amounts of CCL2 and IL-12 could exist. We confirmed this hypothesis by showing that exogenous addition of CCL2 to MD-DC cultures exposed to TLR agonist combination significantly inhibits the synergistic up-modulation of IL-12 secretion, although it does not affect the single agonist-induced IL-12 production. Furthermore, we report for the first time that addition of exogenous CCL2 to DC-T cell cocultures strongly reduces IFN-γ production with respect to untreated control cultures. CCL2 represents a key factor for coordinating inflammatory responses during infection. However, several lines of evidence indicate that CCL2 might also influence T-cell immunity. In keeping with our results, it has been shown that some chemoattractants, including CCL2, can affect IL-12 production by human monocytes and DCs, although these effects, mostly inhibitory, were more dramatic in monocytes/macrophages than DCs.15,39,40 In addition, CCL2 expression is associated with the development of polarized Th2 responses,47,48 and enhances the secretion of IL-4 by T cells.49 In this regard, it is noteworthy that in immune-mediated diseases, exhibiting a Th2 character, CCL2 is expressed at high levels and its neutralization in animal models ameliorates disease.50 Lastly, it has been reported that mice genetically deficient for CCL2 fail to mount Th2 responses.17 Accordingly, our results clearly show that down-regulation of CCL2 secretion favors IL-12 production requested for an optimal Th type 1 polarizing program activation. However, under these experimental conditions, CCL2 neither favors the production of IL-4 nor modulates IL-10 production, indicating that suppression of IFN-γ production is an IL-10–independent effect. Our results are consistent with the hypothesis that CCL2 might control the extent of Th type 1 polarization induction rather than per se promoting a shift toward Th type 2 responses. This effect most likely relies on a direct negative effect of CCL2 on IL-12 expression, that in turns could influence IFN-γ expression in T lymphocytes.
The mechanisms by which engagement of selected TLR pairs may lead to different outcomes, either synergy or inhibition, remain to be established. Type I IFN induction has been demonstrated to play an important role in the cascade of gene expression after TLR signaling.29,30 In this regard, it has been previously reported that an autocrine loop of type I IFN is required for bioactive IL-12p70 secretion by myeloid DCs.26 In our study, we extended this observation to CCL2 by demonstrating that its secretion is not a direct effect of TLR triggering, involving early secretion of IFN-β, but not of TNF-α. However, type I IFN is not the response element that accounts for TLR interference, because it is synergistically up-modulated in MD-DCs exposed to agonist combination. Furthermore, we excluded that inhibition of CCL2 was due to the IL-10 synergistically induced under these experimental conditions, because its neutralization completely failed to rescue CCL2 production.
In this study, we provide evidence that the reduced production of CCL2 observed upon stimulation of MD-DCs with agonist combination mostly occurs at the transcriptional level. Consistently with this observation, we found that despite a clear activation of NF-κB p65 subunit upon exposure of MD-DCs to single TLR agonists, a reduction of p65-binding activity is observed after simultaneous triggering of TLR4 and TLR8. Overall, these results point to molecular effectors, rather than soluble mediators, as possible mechanisms for this regulation.
Based on these assumptions, we performed miR profiling in human myeloid MD-DCs. A panel of miR was found to be modulated after treatments with LPS, R848, or their combination with respect to control MD-DCs. Interestingly, the expression of some miR, including miR-146a, -638, -663, and -923, was found to be differentially modulated in MD-DCs stimulated with TLR agonist combination with respect to single agonists. Although further studies are needed to directly prove the involvement of these differentially expressed miR in down-regulation of CCL2 secretion, our findings may serve as a broad blueprint and resource for future hypothesis generation and hypothesis-driven studies of the role of miR in DC-driven immune responses. Furthermore, our results paint a picture of complex and overlapping patterns of regulation, because the expression of at least some miR can be in turn controlled through TLR-triggered signaling pathways. In this regard, it is noteworthy that according to the observed NF-κB p65 activation impairment, we also found that the expression of the miR-146a, whose expression is controlled by NF-κB, is also reduced.51 Of note, miR-146a/b have been proposed as novel negative regulators contributing to the fine tune of immune response.51
Inappropriate activation of CCL2 expression has been associated with the pathogenesis of several autoimmune and inflammatory diseases,14 and the importance of aberrant miR expression in autoimmune diseases is beginning to emerge.52 Taken together, our findings suggest that selective down-regulation of the proinflammatory chemokine CCL2 can represent a novel regulatory mechanism that may have evolved to maintain immunologic balance. Understanding the combinatorial code by which DCs discriminate pathogens is critical for generating an optimal cytokine response in combating immunomediated diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Patrizia Puddu for help in T-cell polarization experiments; Massimo Delle Femmine for editorial assistance; and Centro Trasfusionale-University of Rome “Sapienza” and Pierre Eid for kindly providing human blood buffy coats and the anti-IFNAR1 mAb 64G12, respectively.
This work was supported by research funds from the European Commission FP6 “Network of Excellence” initiative under contract no. LSHB-CT-2004-512074 DC-THERA, the “Associazione Italiana per la Ricerca sul Cancro,” the Accordo di Collaborazione Italy-USA (to S.G.), and the Italian Ministry of Health (to G.F.B.).
Authorship
Contribution: M.D. and A. Michienzi designed the research, performed the experiments, and contributed to manuscript writing; A. Masotti and L.D. performed the microarray analysis of miR profiling; F.B. and G.F.B. contributed to the intellectual work; and S.G. coordinated the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandra Gessani, Department of Cell Biology and Neurosciences, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161, Rome, Italy; e-mail: sandra.gessani@iss.it.
References
Author notes
*M.D. and A. Michienzi contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal