Abstract
Data from the Italian Hemophilia Centres were collected to perform a retrospective survey of joint arthroplasty in patients with severe hemophilia. Twenty-nine of 49 hemophilia centers reported that 328 of the 347 operations were carried out in 253 patients with severe hemophilia A (HA) and 19 in 15 patients with severe hemophilia B (HB). When results were normalized to the whole Italian hemophilia population (1770 severe HA and 319 severe HB), patients with HA had a 3-fold higher risk of undergoing joint arthroplasty (odds ratio [OR], 3.38; 95% confidence interval [CI], 1.97-5.77; P < .001). These results were confirmed after adjustment for age, HIV, hepatitis C virus (HCV), and inhibitor in a Cox regression model (HR, 2.65; 95% CI, 1.62-4.33; P < .001). The survival analysis of time to joint arthroplasty in the subset of patients with severe HA was not affected by the severity of factor VIII (FVIII) gene mutations. A systematic review of literature articles reporting joint arthroplasties in HA and HB showed that the proportion of HA patients who had undergone arthroplasties was higher than that of HB patients, in agreement with the findings in our Italian cohort. These data suggest that the 2 inherited coagulation disorders have a different severity of clinical phenotype.
Introduction
Chronic arthropathy is the epitome of the complications that occur during the natural history of hemophilia A (HA) and hemophilia B (HB). In the past 3 decades joint arthroplasty was carried out more and more frequently to improve the quality of life of these patients, particularly for the control of severe joint pain. Generally, no difference is thought to exist in terms of frequency of the indication for arthroplasty with respect to the type of hemophilia, because the 2 inherited coagulation defects are considered clinically identical provided the degree of factor deficiency is the same. However, it has been suggested that HA and HB may be different in terms of severity of the bleeding tendency. As early as 1959 Armand Quick1 noticed that HB, even in its severe form, is less handicapping than HA, especially beyond adolescence. More recently a preliminary report by Pai et al2 found that patients with HA bled more often and used more factor concentrates than those with HB with comparable plasma factor levels. A Canadian survey described a smaller prophylactic use of factor IX (FIX) concentrates in patients with hemophilia B (17% vs 53%),3 hypothesizing that this difference may be due, among many other possible causes, to a different clinical severity. Moreover, Schulman et al,4 in the frame of the validation of a composite score meant to assess the clinical severity of hemophilia, found that HA is more severe than HB at the same level of plasma factor deficiency.
The Italian Hemophilia Centres Association (AICE) recently planned a retrospective national data collection on patients who underwent joint arthroplasty. The original scope was to better understand the outcome of these operations and to provide more focused recommendations for treatment and follow-up. We subsequently chose to compare the rates of this type of surgery in patients with HA and HB, taking into account potential confounders such as genotypes, bloodborne infections with the HIV and hepatitis C virus (HCV), and presence of inhibitors. Finally, the results of our study were compared with those of a systematic review of the corresponding data from the literature. The main goal of the systematic review was to limit the risk of selection bias inherent in our retrospective study by comparing the uneven distribution of patients with HA and HB in the Italian cohort with other similar reports.
Methods
Study design
The study, designed as a retrospective cohort study plus a systematic review of the literature, was proposed to the Italian Association of Hemophilia Centres (AICE) and carried out within the framework of the hemophilia treatment centers (HTCs) affiliated to AICE. The study was investigator initiated, and there was no funding or sponsoring.
Retrospective cohort study
All the HTCs were asked to participate in the survey. Those who had performed orthopedic operations between 1987 and 2007 were asked to supply the number of patients who underwent joint arthroplasty and to fill in a case record form with information on the type and severity of hemophilia, birth date, type, site and date of surgery, criteria for surgical indication, and patient identifier for the Italian Registry of Hemophilia and Allied Disorders.5 The record set relative to the patients who underwent joint arthroplasty was then matched with the national database, to allow extraction of data on demography, inhibitor status, viral serology, and genotype. According to the genetic defect patients were further subdivided in null mutations (intron 22 and 1 inversions, large deletions, nonsense, small deletions/insertions not in frame) and non-null mutations (small deletions/insertions in frame, splicing, missense).
Systematic review of the literature
All the published papers that had described cohorts of patients, case series, and case reports of joint arthroplasty in both HA and HB patients were considered. A computerized search of MEDLINE (keywords: hemophil*, haemophil*, joint surg*, orthop*, arthroplast*) was performed. The search was censored on August 2008. In addition, published studies were identified through personal communications, through the hand-scanning of meeting proceedings (World Federation of Hemophilia, International Society on Thrombosis and Hemostasis, American Society of Hematology, European Hematology Association) and reference list of relevant studies. When necessary, particularly to exclude duplicate publications, the authors of the selected papers were called to obtain additional information. Two of us (G.T. and A.I.) independently reviewed and extracted data using a standard form, including number of patients with HA and HB who underwent joint arthroplasty and the type of prosthesis.
Statistical analysis: retrospective cohort study
The main outcome of the study was the comparison of the raw rates (prevalence, expressed as percentage) of joint arthroplasty among Italian patients with HA and HB. Rates were calculated both on patient and joint bases. The total number of patients and the number of operations for each joint were divided by the whole population of patients with HA or HB included in the Italian National Registry. The resulting rates were then compared with the chi-squared statistics. Odds ratios (OR) and 95% confidence intervals (CI) were calculated as indicators of relative risks of arthroplasty with the method of Mantel-Henszel. Finally, the combined dataset of the arthroplasty survey and of the national database were investigated for potential confounders by using a survival analysis model and estimating the hazard ratio to undergo prosthetic surgery for HA versus HB. A Cox regression model was used to adjust for such covariates as inhibitor status, HCV and HIV infections, and type of genetic mutation.
Systematic review of the literature
Because of the unavailability of accurate information on the percentages of HA and HB in the different hemophilia populations from which the published arthroplasty case series were extracted, we could not calculate nor compare the rate of arthroplasties in patients with HA versus patients with HB. Hence, we chose, as outcome of the analysis, the percentage of joint arthroplasties in patients with HA and HB in the whole arthroplasty series, calculated with the formula: [arthroplasties in HA/(arthroplasties in HA + arthroplasties in HB)] × 100. This percentage was recalculated for each study, as well as for the whole Italian cohort, together with 95% CIs that were calculated assuming a binomial distribution. Then a weighted pooled percentage was obtained, using as a weight variable the reversal of the variance of the studies.6 A fixed effect model was planned at first; if significant heterogeneity was found with the Cochran Q test, a random-effect model was then used. Finally, the observed percentage of patients with HA in the arthroplasty series stemming from the systematic review was compared with the percentage of HA in the population of hemophiliacs, estimated as the pooled mean percentage of HA in the Italian, Canadian, and United Kingdom national hemophilia databases.5 The chi-squared statistics was used to test for the difference between observed and expected percentage. All the calculations were performed with STATA version 9 for Windows (Stata Corporation).
Results
Retrospective cohort study
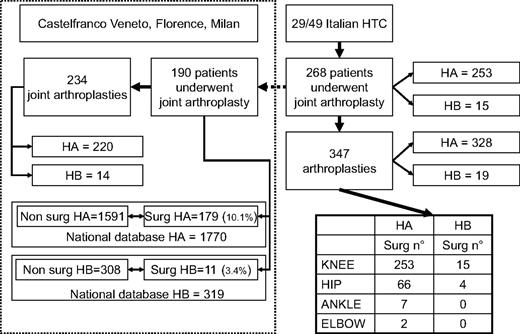
Twenty-nine HTCs in Italy provided the records of 268 patients who underwent 347 operations of joint arthroplasty between January 1987 and December 2007. The remaining 20 centers did not perform this surgery. Figure 1 shows the flow chart of the study dataset. Three hundred twenty-eight joint arthroplasties were performed in patients with HA, 19 in patients with HB. Among the 347 joints replaced in both hemophilias, 268 were knees, 70 hips, 7 ankles, and 2 elbows. Figure 1 also shows the distribution of arthroplasties between HA and HB. Arthroplasties were performed in 268 patients, 253 HA and 15 HB.
Flowchart of the Italian cohort patients who underwent arthroplasty. The diagram on the left shows the flow chart of the data for the whole Italian arthroplasty dataset. On the left, the area inside the dotted line shows details of the subset of patients who underwent arthroplasty in 1 of the 3 main hemophilia centers; availability of the full records of the subset allowed matching with the Italian Registry of Hemophilia to perform the time-to-event analysis (see “Results”). The table at the bottom right shows details about arthroplasty distribution by joint. The corresponding rates in the Italian hemophilia population are given in the text.
Flowchart of the Italian cohort patients who underwent arthroplasty. The diagram on the left shows the flow chart of the data for the whole Italian arthroplasty dataset. On the left, the area inside the dotted line shows details of the subset of patients who underwent arthroplasty in 1 of the 3 main hemophilia centers; availability of the full records of the subset allowed matching with the Italian Registry of Hemophilia to perform the time-to-event analysis (see “Results”). The table at the bottom right shows details about arthroplasty distribution by joint. The corresponding rates in the Italian hemophilia population are given in the text.
The rate of patients undergoing arthroplasty in the whole population of Italian hemophiliacs was calculated, taking as denominator the numbers stemming from the National Registry, that is, 1770 severe HA and 319 severe HB. Two hundred fifty-three of 1770 severe HA (14.3%; 95% CI, 12.7%-15.9%) and 15 of 319 severe HB (4.7%; 95% CI, 2.4%-7.0%) underwent arthroplasty. The risk of undergoing arthroplasty expressed as an OR was 3.38 (95% CI, 1.97-5.77; P < .001) when patients were considered. When single joints were considered, 328 operations were performed in 1770 patients with severe HA (18.5%), 19 in 319 patients with severe HB (6.0%), with an OR 3.59 (95% CI, 2.22-5.80; P < .001). The risk of undergoing knee arthroplasty expressed as an OR was 3.38 (95% CI, 1.97-5.77; P < .001) and that for hip arthroplasty was 3.05 (95% CI, 1.10-8.43; P = .032) for HA compared with HB.
Figure 1 shows the detailed case record forms that could be obtained for 190 of the 286 whole patient population (70%, 179 HA and 11 HB patients), all severe and all undergoing arthroplasty in the largest HTCs of Castelfranco Veneto, Florence, or Milan (Figure 1). This cohort of patients undergoing surgery included not only patients regularly followed by these centers, but also those referred for orthopedic surgery from other Italian centers. The indication for surgery was the same in all cases, ie, severe joint pain poorly controlled by analgesic drugs. Patients were analyzed in the frame of the whole cohort of 2089 Italian patients with severe hemophilia (1770 HA and 319 HB). At the time of the analysis, 177 of 190 patients undergoing joint arthroplasty were alive (93.1%; 167 HA and 10 HB); similarly, 1785 of 1899 nonsurgical patients were alive (94.0%; 1535 HA and 250 HB). Age distribution of the patients alive is shown in Table 1. Both the patients with HA and the patients with HB who had undergone joint arthroplasty were significantly older than those who did not and were born with severe hemophilia in the 1960s, at a time when replacement therapy was not available on a regular basis in Italy, neither for HA nor for HB. The OR of joint arthroplasty for HA or HB was 3.15 (95% CI, 1.69-5.86) for the whole cohort and 2.93 (95% CI, 1.52-5.63) for patients alive.
Age of patients alive at the time of analysis by hemophilia type and arthroplasty surgery
| . | Hemophilia A . | Hemophilia B . | All . |
|---|---|---|---|
| No surgery | 28.8 ± 17.0 (28) | 27.4 ± 17.1 (25) | 28.6 ± 17.0 (28) |
| Surgery | 45.7 ± 10.2 (44) | 45.5 ± 13.4 (43) | 45.6 ± 10.4 (44) |
| All | 30.7 ± 17.2 (31) | 28.0 ± 17.3 (26) |
| . | Hemophilia A . | Hemophilia B . | All . |
|---|---|---|---|
| No surgery | 28.8 ± 17.0 (28) | 27.4 ± 17.1 (25) | 28.6 ± 17.0 (28) |
| Surgery | 45.7 ± 10.2 (44) | 45.5 ± 13.4 (43) | 45.6 ± 10.4 (44) |
| All | 30.7 ± 17.2 (31) | 28.0 ± 17.3 (26) |
Age is in years. Mean ± SD (median) were calculated.
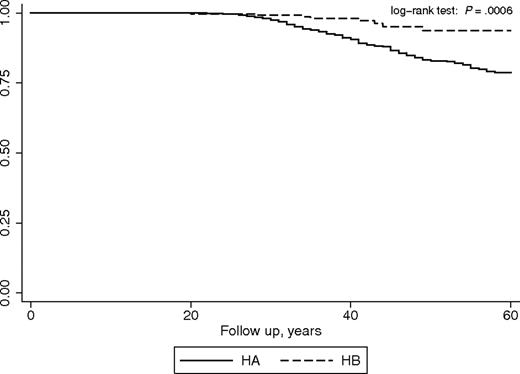
To assess whether the odds for joint arthroplasty were confounded by other patient characteristics, a survival time analysis was performed, estimating the crude and adjusted hazard ratios of undergoing joint arthroplasty for patients with HA versus those with HB (taken as reference group). The survival analysis per se adjusts for differences in mortality rates; other potentially relevant variables included in the model were HIV, HCV, and inhibitor status. Regular prophylaxis was not included among the variables, because neither patients with HA nor patients with HB were on regular primary prophylaxis during their lifetime before arthroplasty. There were 328 inhibitors (322 HA and 6 HB), 386 HIV positive (332 HA and 54 HB), and 1047 HCV positive (903 HA and 144 HB) patients in the dataset. Nineteen inhibitor patients had undergone joint arthroplasty (15 knee and 4 hip, all HA). The unadjusted Kaplan-Meier analysis gave a statistically significant log-rank test (P < .001), and the relative hazard ratio was 3.03 (95% CI, 1.87-4.91; P < .001; Figure 2). Cox regression analysis showed that none of the aforementioned covariates was statistically significant in the model, and the adjusted hazard ratio was 2.65 (95% CI, 1.62-4.33; P < .001).
Joint arthroplasty-free survival for patients with HA and patients with HB. The curves show the crude proportion of patients who have not yet undergone surgery at any age time point.
Joint arthroplasty-free survival for patients with HA and patients with HB. The curves show the crude proportion of patients who have not yet undergone surgery at any age time point.
Table 2 reports the genetic defects extracted from the Italian molecular database for HA and HB.7,8 HA mutations were available for a total of 1206 patients (1086 nonsurgery and 120/179 surgery), whereas for HB the known mutations were 235 (224 nonsurgery and all 11 surgery). The percentage of null mutations was identical in patients with HA, whether undergoing arthroplasty (79% vs 76%), and the risk of undergoing surgery for HA was not related to the type of mutation at Cox regression (HR = 0.92; 95% CI, 0.59-1.43). No survival analysis was feasible for patients with HB, but the percentage of patients with null mutations was higher in arthroplasty (55%) compared with nonarthroplasty patients (28%).
Distribution of genetic mutations
| . | Severe HA with available genotype . | Severe HB with available genotype . | ||
|---|---|---|---|---|
| Nonsurgery patients (n = 1086) . | Surgery patients (n = 120) . | Nonsurgery patients (n = 225) . | Surgery patients (n = 11) . | |
| Null mutations | 820 | 95 | 62 | 6 |
| Intron 22/intron 1 INV | 533 | 65 | / | |
| Large deletions | 13 | 2 | 9 | |
| Nonsense | 101 | 13 | 42 | 6 |
| Small del/ins not in frame | 153 | 15 | 10 | |
| Non-null mutations | 202 | 25 | 162 | 5 |
| Small del/ins in frame | 11 | 2 | 19 | |
| Splicing | 24 | 3 | 14 | 1 |
| Missense | 167 | 20 | 129 | 4 |
| Not identified | 64 | / | ||
| Null mutation (%) | 76 | 79 | 28 | 55 |
| 95% confidence interval (%) | 73-78 | 72-76 | 22-33 | 25-84 |
| . | Severe HA with available genotype . | Severe HB with available genotype . | ||
|---|---|---|---|---|
| Nonsurgery patients (n = 1086) . | Surgery patients (n = 120) . | Nonsurgery patients (n = 225) . | Surgery patients (n = 11) . | |
| Null mutations | 820 | 95 | 62 | 6 |
| Intron 22/intron 1 INV | 533 | 65 | / | |
| Large deletions | 13 | 2 | 9 | |
| Nonsense | 101 | 13 | 42 | 6 |
| Small del/ins not in frame | 153 | 15 | 10 | |
| Non-null mutations | 202 | 25 | 162 | 5 |
| Small del/ins in frame | 11 | 2 | 19 | |
| Splicing | 24 | 3 | 14 | 1 |
| Missense | 167 | 20 | 129 | 4 |
| Not identified | 64 | / | ||
| Null mutation (%) | 76 | 79 | 28 | 55 |
| 95% confidence interval (%) | 73-78 | 72-76 | 22-33 | 25-84 |
All Italian patients with available genotype are reported. The HA surgery patients with known mutation are 120 of the 179 patients from the 3 main hemophilia centers. All the 11 patients with HB are included.
Systematic review of the literature
The literature search yielded 7 studies9-15 published between 1983 and 2007. Altogether, those studies described 165 patients who underwent joint arthroplasty. After exclusion of 4 patients repeated in 2 reports11,14 161 patients were included in the review. The patients were 147 HA and 14 HB, mostly severely affected and mostly undergoing knee or hip replacement, or both. Relevant details of the studies are given in Table 3. The table also shows the percentage of HA in the case series of patients undergoing joint arthroplasty in each study. To obtain an overall estimate of the percentage of patients with HA in the population undergoing joint arthroplasty, we first analyzed the studies with a classical fixed effect model. Intertrial heterogeneity was not significant, allowing the pooling procedure. The overall estimate of the percentage of patients with HA from the literature data were 89.5% (95% CI, 83.0%-93.7%), that overlapped with the percentage found in our case series, which was 94.5% (95% CI, 91.6%-96.5%). Thus, the overall pooled percentage of patients with HA in the population undergoing joint arthroplasty in the systematic review of both the literature and Italian series yielded a final value of 92.8% (95% CI, 90.1%-94.9%). The expected percentage of patients with HA, calculated using the weighted mean of the Italian, Canadian, and United Kingdom databases, was 84.0% (95% CI, 81.7%-86.3%). The difference between the observed and expected percentages was statistically significant (Pearson χ2 = 23.54, P < .001), showing an uneven distribution of arthroplasties between patients with HA and patients with HB.
Published data on joint arthroplasties in hemophilic patients
| Reference . | No. of arthroplasties . | HA patients, n . | HB patients, n . | Arthroplasties in HA, % . | 95% CI . |
|---|---|---|---|---|---|
| Small et al9 | 5 TKR | 4 | 1 | 80.0 | 30.9-97.3 |
| Magone et al10 | 9 TKR | 6 | 1 | 85.7 | 41.9-98.0 |
| Kelley et al11 | 34 THR | 26 | 1 | 96.3 | 77.9-99.5 |
| Heeg et al12 | 12 TJR | 7 | 1 | 87.5 | 46.3-98.3 |
| Thomanson et al13 | 23 TKR | 13 | 2 | 86.7 | 59.5-96.6 |
| Hicks et al14 | 102 TJR | 66* | 3 | 95.9 | 88.0-98.7 |
| Rodriguez-Merchan15 | 34 TKR | 25 | 5 | 83.3 | 65.7-92.9 |
| Tagariello et al, this study | 328 TJR | 235 | 15 | 94.4 | 90.9-96.6 |
| Reference . | No. of arthroplasties . | HA patients, n . | HB patients, n . | Arthroplasties in HA, % . | 95% CI . |
|---|---|---|---|---|---|
| Small et al9 | 5 TKR | 4 | 1 | 80.0 | 30.9-97.3 |
| Magone et al10 | 9 TKR | 6 | 1 | 85.7 | 41.9-98.0 |
| Kelley et al11 | 34 THR | 26 | 1 | 96.3 | 77.9-99.5 |
| Heeg et al12 | 12 TJR | 7 | 1 | 87.5 | 46.3-98.3 |
| Thomanson et al13 | 23 TKR | 13 | 2 | 86.7 | 59.5-96.6 |
| Hicks et al14 | 102 TJR | 66* | 3 | 95.9 | 88.0-98.7 |
| Rodriguez-Merchan15 | 34 TKR | 25 | 5 | 83.3 | 65.7-92.9 |
| Tagariello et al, this study | 328 TJR | 235 | 15 | 94.4 | 90.9-96.6 |
TKR indicates total knee replacement; THR, total hip replacement; TJR, total joint replacement (joint unspecified); CI, confidence interval; HA, hemophilia A; and HB, hemophilia B.
The study originally reported 70 HA patients, but 4 were excluded because they were duplicated in the study by Kelley et al.
Discussion
Even though severe HA and HB are classically considered identical from a clinical standpoint, the results of this study provide evidence that the risk of undergoing joint arthroplasty is different for the 2 inherited coagulation disorders. The risk of patients with HA needing an arthroplasty was 3-fold higher, with no differences for the sites of prosthesis, but the same proportion was seen for knee or hip in both diseases. The small number of ankle and elbow arthroplasties permits no analysis, but they were all in patients with HA.
Factors other than the type of coagulation defect that might influence the natural course of the disease and survival rate were evaluated, such as inhibitors, HCV and HIV infections. In particular, although the prevalence of HIV infection is 26% among Italian patients with HA who are currently living, the corresponding figure is 47.1% in those alive with HB.16 Excess rate of mortality may have reduced the number of patients with HB at risk for joint arthroplasty, thus biasing our analysis. However, Cox regression analysis found that this and other potential confounders had no significant role in the model. Moreover, although the presence of inhibitors might introduce bias as they were all in HA, the presence of this complication generally discourages clinicians from performing elective orthopedic surgery. Thus, bias should play against our hypothesis that the type of coagulation defect affects the need for joint arthroplasty.
This retrospective study has several limitations, such as the inaccurate knowledge of the detailed history of patients, including yearly bleeding frequency. Failure to capture all data on patients and joint arthroplasties and inaccurate diagnosis of hemophilia severity are other possible limits, implicit in any retrospective survey, but there is no reason to think that these possible inaccuracies were different in HA and HB. By the same token, it cannot be established with certainty whether the severity of pain, taken as the main indication for joint arthroplasties, was uniformly assessed in the 3 main operating centers during the 10-year period or whether the other referring centers truly used the same criteria for surgical indications. However, another important source of bias, ie, heterogeneity of replacement therapy regimens for HA versus HB in different centers at different times, appears unlikely, because the average age of our patients is 45 years. In Italy no patient had been put on primary prophylaxis before the 1990s, and regular treatment on demand with factor VIII (FVIII) or prothrombin complex concentrates started not earlier than in the 1970s for both hemophilias.
We attempted to replicate our results by comparing them with those of the hemophilia arthroplasty literature. Only a minority of reports provide detailed data on the type of hemophilia. Yet we were able to identify 7 reports9-15 that described both the type and severity of hemophilia and the type of arthroplasty performed. However, at variance with our own study, the proportions of patients with HA or HB from the total population with hemophilia could not be obtained from these reports. Hence, results could only be expressed as percentage of arthroplasties in HA versus those in HA plus HB, yielding a value of 89.7% (95% CI, 83.2%-93.8%) that perfectly overlaps with that found in our case series using the same calculation criteria (Table 3). However, it must be considered that all the studies included in our systematic review were retrospective (case reports and case series), so that they carry a definite risk of selection bias which is not reduced by the meta-analytic process. The contribution of the systematic review is in showing that the possible selection bias was homogeneous among all the reports, which makes it rather unlikely.
Assuming that the different rates of prosthetic surgery in HA and HB reflects a lower degree of clinical severity for the latter leading to a lower risk of severe arthropathy, there are several possible reasons for this difference. One is that HB is caused by less severe gene mutations than HA because, as also shown by the Italian database of mutations,7 the majority of HB cases is caused by point mutations (missense), with only a relatively small proportion of null mutations (eg, large deletions, nonsense mutations, or rearrangements). However, in severe HA more than half of the cases are caused by null mutations such as intron 22 inversion (40%-50%) and intron 1 inversion (3%-5%).8 These and other null mutations, such as large deletions and nonsense mutations, do not permit FVIII synthesis. In addition, more patients with HB than with HA are cross-reacting material positive (CRM+), with measurable plasma levels of FIX antigen.17 We found a higher percentage of null mutations in patients with HB undergoing arthroplasty (55%) than in those not undergoing surgery (28%). However, when the entire cohort of Italian patients with severe HA undergoing arthroplasty was analyzed according to the type of gene mutation, the proportion of null mutations in patients with HA undergoing surgery was 79%, that is, identical to that of 76% observed in patients without surgery. Actually, the survival analysis of time to surgery of this subset of patients with HA showed no effect of the severity of the underlying mutation on the risk to undergo arthroplasty. Thus, even if the severity of the underlying mutation does contribute to explain the difference between HA and HB, other mechanisms should be considered. As an example, a recent observation in an animal model showed the protective role of factor IX injected into the joint space, in the absence of measurable circulating plasma FIX activity, against the development of synovitis.18 Perhaps very low levels of plasma FIX, which are currently undetectable because of the limited sensitivity of available assays of functional activity, play a protective role in the development of the chronic arthropathy in hemophilia B. Global coagulation assays such as the thromboelastogram and the thrombin potential may perhaps be able to show different amounts or dynamics of thrombin generation in the 2 hemophilias.
In conclusion these data on the different rates of joint arthroplasty, the epitome of the orthopedic operations that reflects the degree and severity of hemophilic arthropathy, do suggest a different severity of the clinical phenotype between the 2 inherited coagulation disorders. Our findings may have potential clinical implications, because they add another piece of evidence to previous observations that HB is less severe than HA.1-4 Perhaps this would direct clinicians to plan less primary prophylaxis in HB, or at least to adopt escalating prophylaxis regimens, that would start with weekly concentrate infusion and increase infusion frequency only when breakthrough bleedings occur.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Maurizio Margaglione from the Genetica Medica, Università di Foggia for the HA mutation database; Donata Belvini, who is a fellow of the Associazione Progresso Ematologico (APE); and Roberta Salviato from the Transfusion Service, Hemophilia and Regional Blood Disease Center, Castelfranco Veneto for the HB database and interpretation of the mutations. We thank Scott Kelley, from the North Carolina Orthopaedic Clinic, who provided information on duplicated patients.
Authorship
Contribution: G.T., A.I., and P.M.M. designed the research, analyzed data, and wrote the manuscript; E.S., M.M., and M.E.M. designed the research, enrolled patients, and reviewed the manuscript; and R.B., M.I., M.G.M., G.L.P, P.R., G.R., C.S., R.S., A.S. and L.P.S. enrolled patients, participated in discussion, and provided general support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Italian Association Hemophilia Centre (AICE) appears in the online Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Giuseppe Tagariello, Castelfranco Veneto Hospital, Via Ospedale 18, 31033 Castelfranco Veneto (TV), Italy; e-mail: giuseppe.tagariello@ulssasolo.ven.it.