Abstract
We examined whether monoclonal gammopathy of undetermined significance (MGUS) is increased in first-degree relatives of multiple myeloma (MM) or MGUS patients. Probands were recruited from a population-based prevalence study (MGUS) and the Mayo Clinic (MM). Serum samples were collected from first-degree relatives older than 40 years and subjected to electrophoresis and immunofixation. The prevalence of MGUS in relatives was compared with population-based rates. Nine-hundred eleven relatives of 232 MM and 97 MGUS probands were studied. By electrophoresis, MGUS was detected in 55 (6%) relatives, and immunofixation identified 28 additional relatives for an age- and sex-adjusted prevalence of 8.1% (95% CI, 6.3 to 9.8). The prevalence of MGUS in relatives increased with age (1.9%, 6.9%, 11.6%, 14.6%, 21.0% for ages 40-49, 50-59, 60-69, 70-79, ≥ 80 years, respectively; P < .001). Using similar MGUS detection methods, there was a higher risk of MGUS in relatives (age-adjusted risk ratio [RR], 2.6; 95% CI, 1.9 to 3.4) compared with the reference population. The increased risk was seen among relatives of MM (RR, 2.0; 95% CI, 1.4 to 2.8) and MGUS probands (RR, 3.3; 95% CI, 2.1 to 4.8). The increased risk of MGUS in first-degree relatives of MGUS or MM patients implies shared environment and/or genetics.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is the most common plasma cell proliferative disorder, prevalent in approximately 3% of the general population older than 50 years.1,2 The prevalence of MGUS increases with age from 1.7% in those 50 to 59 years of age to more than 5% in those older than 70 years. Within each age group, prevalence is higher in men than in women. Moreover, the age-adjusted prevalence of MGUS is 3-fold higher in blacks from Ghana and in African Americans compared with whites.3-5 In contrast, the age-adjusted prevalence appears to be lower in Japan.6 MGUS is associated with an increased risk of multiple myeloma (MM) or related malignancy at a rate of 1% per year.1 The rate of progression of MGUS to myeloma remains constant over time, consistent with a simple random multihit genetic model of malignancy. Thus, it is likely that certain inherited genetic factors may predispose patients to developing MGUS, thereby initiating the cascade of events toward MM. However, the risk of MGUS in close family members of patients with MM and MGUS is not known. In one study, no increase in the incidence of MGUS was noted among family members of 218 MM cases in Iceland compared with the general Icelandic population, but this study did not screen family members for MGUS.7 Other studies have suggested a possible increased risk of MM in persons with a family history of MM,8-10 but there are no good data on whether there is an increased familial predisposition for MGUS.
The goal of this study was to determine whether the risk of MGUS is increased in first-degree relatives of patients with MM or MGUS. If so, family history would add to the limited number of known risk factors for MGUS, and the results would provide important rationale for studying shared environment and genetic changes underlying this disorder. And, clinically, an increase in the expected baseline rate of MGUS among relatives will have an impact on the treatment of MGUS in these patients when it is incidentally diagnosed during workup for other potentially related disorders.
Methods
The study design and conduct was approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from all participants in this study, in accordance with the Declaration of Helsinki.
Study probands
First-degree relatives for this study were derived from 2 patient populations, comprising probands with MGUS or MM. The first was a well-defined cohort of MGUS patients 50 years of age or older from Olmsted County, MN identified through a population-based prevalence study.2 As previously reported, from January 1, 1995, to December 31, 2001, serum samples were obtained from 21463 (76.6%) of 28038 enumerated Olmsted County residents. MGUS was identified in 694 patients (3.2% of the population). Of the 694, 89 were derived from blinded samples and could not be contacted and 301 were deceased, leaving 304 living MGUS probands eligible for this family study. None of the 304 probands from the original Olmsted County Study were related. These MGUS probands were mailed an invitation letter, consent form, and family history questionnaire, soliciting names and addresses of first-degree (siblings, children, parents) blood-related family members ages 40 years and older.
The second population consisted of MM patients seen at the Mayo Clinic between February 2006 and September 2007. These patients were invited to participate through a letter distributed during their clinical appointment. A consent form and family questionnaire (identical to the one used for MGUS probands) was then mailed to consenting patients.
First-degree relatives
All first-degree blood relatives ages 40 years or older identified by the probands were mailed an invitation and informed consent form. Upon receipt of the completed consent form, a blood collection kit was mailed to the relative for blood to be collected at a local facility and returned to the Mayo Clinic for testing to determine presence of a serum monoclonal protein. Consenting individuals were also invited to complete a short risk factor questionnaire.
Serum protein electrophoresis
All serum samples were processed and analyzed in an identical fashion and in the same laboratory (Mayo Clinic Protein Immunology Laboratory, Rochester, MN) as for the population-based study of MGUS in Olmsted County (reference population)2 to which the MGUS rates in first-degree relatives were to be compared. Serum protein electrophoresis was performed on agarose gel (REP; Helena Laboratories). The agarose strip was inspected by a technician and by 2 of the authors (R.A.K. and J.A.K.) who were blinded to participant characteristics, including family history of MGUS and MM. Any serum with a discrete band or thought to have a localized band was confirmed and typed by serum immunofixation (Hydrasys and Hydragel; Sebia). MGUS was defined in accordance with the standard definition used in the Olmsted County prevalence study.2 The comparisons of prevalence between first-degree relatives and the reference population were based on MGUS cases identified through this diagnostic strategy.
Serum immunofixation and free light chain assay
Besides comparing the incidence of MGUS in first-degree relatives to the reference population (Olmsted County), another goal of the study was to determine the true prevalence of MGUS in first-degree relatives using the most sensitive techniques. Thus, to detect small monoclonal proteins below the level of detection by electrophoresis, samples were also tested by serum immunofixation. In addition, we tested all samples with the serum free light chain (FLC) assay to detect patients with MGUS in whom the immunoglobulin heavy chain expression is lost.
Statistical considerations
Age-specific prevalence was calculated by dividing the number of persons with MGUS in each age and sex stratum by the number of subjects in the strata with an assayed serum sample. Overall sex- and age-adjusted prevalence was obtained by direct standardization to the 2000 US population. Age- and sex-specific rates of MGUS from the population-based Olmsted County prevalence study2 were used as expected rates for the calculation of risk ratios (RRs) for comparisons of all first-degree relatives, and separately for relatives of each proband type (MM or MGUS). These reference rates reflect the prevalence of MGUS among 76.6% of Olmsted County residents 50 years or older, and are generally representative of the white population of the United States.11 Expected rates for the 40- to 49-year-old age group were estimated using linear interpolation assuming a prevalence of zero at age 30 years. In addition, the age-specific prevalence pattern of MGUS was estimated using Poisson regression incorporating a smoothed function of age, using a generalized additive models framework. This method is illustrated in a previously published study of hip fracture incidence.12
The association of the risk of MGUS by age group, sex of relative, relationship to proband, and proband diagnosis characteristics (monoclonal isotype, M-protein size, age at diagnosis) was evaluated using Poisson regression; the number of MGUS cases was entered as the dependent variable, the factor of interest as the independent variable, and the age- and sex-specific expected rates of MGUS were included as an offset.
Results
A total of 304 MGUS probands from the Olmsted County prevalence study and 407 MM probands seen at the Mayo Clinic were invited to participate in the study. Of these, 501 probands (160 with MGUS, and 341 with MM) agreed to participate and provided contact information for their blood-related first-degree relatives 40 years and older. Of 1760 relatives invited (574 of a MGUS proband and 1186 of a MM proband), 247 (43%) relatives of 97 MGUS probands and 664 (56%) relatives of 232 MM probands contributed blood samples and questionnaire data. None of the relatives had previously received a MGUS diagnosis at the Mayo Clinic or were part of the original Olmsted County Screening study. The characteristics of probands and relatives are given in Table 1.
Baseline characteristics of probands and relatives
| Probands, N = 329, no. (%) . | MGUS proband, n = 97 . | MM proband, n = 232 . | Total . |
|---|---|---|---|
| Male | 48 (49.5) | 126 (54.3) | 174 (52.9) |
| Female | 49 (50.5) | 106 (45.7) | 155 (47.1) |
| Age group, y, no. (%) | |||
| Younger than 40* | 0 (0) | 6 (2.6) | 6 (1.8) |
| 40-49 | 0 (0) | 30 (13.0) | 30 (9.2) |
| 50-59 | 20 (20.6) | 79 (34.2) | 99 (30.2) |
| 60-69 | 48 (49.5) | 75 (32.5) | 123 (37.5) |
| 70-79 | 26 (26.8) | 38 (16.5) | 64 (19.5) |
| 80+ | 3 (3.1) | 3 (1.3) | 6 (1.8) |
| First-degree relatives, N = 911, no. (%) | MGUS relatives, n = 247 | MM relatives, n = 664 | Total |
| Male | 109 (44.1) | 273 (41.1) | 382 (41.9) |
| Female | 138 (55.9) | 391 (58.9) | 529 (58.1) |
| Relationship to proband | |||
| Parent | 1 (0.4) | 93 (14.0) | 94 (10.3) |
| Sibling | 101 (40.9) | 392 (59.0) | 493 (54.1) |
| Child | 145 (58.7) | 179 (27.0) | 324 (35.5) |
| Age group, y, no. (%) | |||
| 40-49 | 57 (23.1) | 201 (30.3) | 258 (28.3) |
| 50-59 | 76 (30.8) | 157 (23.6) | 233 (25.6) |
| 60-69 | 47 (19) | 143 (21.5) | 190 (20.9) |
| 70-79 | 39 (15.8) | 91 (13.7) | 130 (14.3) |
| 80+ | 28 (11.3) | 72 (10.8) | 100 (11) |
| Probands, N = 329, no. (%) . | MGUS proband, n = 97 . | MM proband, n = 232 . | Total . |
|---|---|---|---|
| Male | 48 (49.5) | 126 (54.3) | 174 (52.9) |
| Female | 49 (50.5) | 106 (45.7) | 155 (47.1) |
| Age group, y, no. (%) | |||
| Younger than 40* | 0 (0) | 6 (2.6) | 6 (1.8) |
| 40-49 | 0 (0) | 30 (13.0) | 30 (9.2) |
| 50-59 | 20 (20.6) | 79 (34.2) | 99 (30.2) |
| 60-69 | 48 (49.5) | 75 (32.5) | 123 (37.5) |
| 70-79 | 26 (26.8) | 38 (16.5) | 64 (19.5) |
| 80+ | 3 (3.1) | 3 (1.3) | 6 (1.8) |
| First-degree relatives, N = 911, no. (%) | MGUS relatives, n = 247 | MM relatives, n = 664 | Total |
| Male | 109 (44.1) | 273 (41.1) | 382 (41.9) |
| Female | 138 (55.9) | 391 (58.9) | 529 (58.1) |
| Relationship to proband | |||
| Parent | 1 (0.4) | 93 (14.0) | 94 (10.3) |
| Sibling | 101 (40.9) | 392 (59.0) | 493 (54.1) |
| Child | 145 (58.7) | 179 (27.0) | 324 (35.5) |
| Age group, y, no. (%) | |||
| 40-49 | 57 (23.1) | 201 (30.3) | 258 (28.3) |
| 50-59 | 76 (30.8) | 157 (23.6) | 233 (25.6) |
| 60-69 | 47 (19) | 143 (21.5) | 190 (20.9) |
| 70-79 | 39 (15.8) | 91 (13.7) | 130 (14.3) |
| 80+ | 28 (11.3) | 72 (10.8) | 100 (11) |
MGUS probands were recruited from a prevalence study of residents 50 years and older.
Prevalence of MGUS
Of the combined 911 first-degree relatives of either a MM or MGUS proband who provided a blood sample, MGUS was detected in the serum of 55 (6%) by serum protein electrophoresis, for an age- and sex-adjusted prevalence of MGUS among all relatives of 5.4% (95% CI, 3.9 to 6.8). These 55 relatives were distributed across 52 families, with 3 families having 2 affected members other than the proband. The age-specific prevalence of MGUS for all relatives combined increased with age, from 0.8% for 40- to 49-year-olds to 13% for those 80 years or older (Table 2). Examining relatives of MM and MGUS probands separately, the age- and sex-adjusted prevalence of MGUS was 4.5% (95% CI, 2.9 to 6.1) for relatives of a MM proband and 7.4% (95% CI, 4.2 to 10.7) for relatives of a MGUS proband (Table 2).
Prevalence of MGUS in first-degree relatives of MGUS and myeloma (MM) probands compared with Olmsted County, MN residents
| Age, y . | All relatives . | Relatives of MGUS proband . | Relatives of MM proband . | Olmsted County . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. . | No. with MGUS . | Prevalence (95% CI) . | Total No. . | No. with MGUS . | Prevalence (95% CI) . | Total No. . | No. with MGUS . | Prevalence (95% CI) . | TotalNo. . | No. with MGUS . | Prevalence (95% CI) . | |
| 40-49 | 258 | 2 | 0.8 (0.1-2.8) | 57 | 0 | 0 (0.0-6.2) | 201 | 2 | 1.0 (0.1-3.5) | |||
| 50-59 | 233 | 11 | 4.7 (2.4-8.3) | 76 | 5 | 6.6 (2.2-14.7) | 157 | 6 | 3.8 (1.4-8.1) | 8373 | 141 | 1.7 (1.4-2.0) |
| 60-69 | 190 | 14 | 7.4 (4.1-12.1) | 47 | 3 | 6.4 (1.3-17.5) | 143 | 11 | 7.7 (3.9-13.3) | 6019 | 178 | 3.0 (2.5-3.4) |
| 70-79 | 130 | 15 | 11.5 (6.6-18.3) | 39 | 8 | 20.5 (9.3-36.5) | 91 | 7 | 7.7 (3.1-15.2) | 4508 | 205 | 4.6 (4.0-5.2) |
| 80+ | 100 | 13 | 13.0 (7.1-21.2) | 28 | 6 | 21.4 (8.3-41.0) | 72 | 7 | 9.7 (4.0-19.0) | 2563 | 170 | 6.6 (5.7-7.7) |
| Total, adjusted* | 911 | 55 | 5.4 (3.9-6.8) | 247 | 22 | 7.4 (4.2-10.7) | 664 | 33 | 4.5 (2.9-6.1) | 21463 | 694 | 3.2 (3.0-3.5) |
| Total, adjusted† | 911 | 83 | 8.1 (6.3-9.8) | 247 | 30 | 10.9 (6.8-15.0) | 664 | 53 | 7.0 (5.1-9.0) | |||
| Age, y . | All relatives . | Relatives of MGUS proband . | Relatives of MM proband . | Olmsted County . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. . | No. with MGUS . | Prevalence (95% CI) . | Total No. . | No. with MGUS . | Prevalence (95% CI) . | Total No. . | No. with MGUS . | Prevalence (95% CI) . | TotalNo. . | No. with MGUS . | Prevalence (95% CI) . | |
| 40-49 | 258 | 2 | 0.8 (0.1-2.8) | 57 | 0 | 0 (0.0-6.2) | 201 | 2 | 1.0 (0.1-3.5) | |||
| 50-59 | 233 | 11 | 4.7 (2.4-8.3) | 76 | 5 | 6.6 (2.2-14.7) | 157 | 6 | 3.8 (1.4-8.1) | 8373 | 141 | 1.7 (1.4-2.0) |
| 60-69 | 190 | 14 | 7.4 (4.1-12.1) | 47 | 3 | 6.4 (1.3-17.5) | 143 | 11 | 7.7 (3.9-13.3) | 6019 | 178 | 3.0 (2.5-3.4) |
| 70-79 | 130 | 15 | 11.5 (6.6-18.3) | 39 | 8 | 20.5 (9.3-36.5) | 91 | 7 | 7.7 (3.1-15.2) | 4508 | 205 | 4.6 (4.0-5.2) |
| 80+ | 100 | 13 | 13.0 (7.1-21.2) | 28 | 6 | 21.4 (8.3-41.0) | 72 | 7 | 9.7 (4.0-19.0) | 2563 | 170 | 6.6 (5.7-7.7) |
| Total, adjusted* | 911 | 55 | 5.4 (3.9-6.8) | 247 | 22 | 7.4 (4.2-10.7) | 664 | 33 | 4.5 (2.9-6.1) | 21463 | 694 | 3.2 (3.0-3.5) |
| Total, adjusted† | 911 | 83 | 8.1 (6.3-9.8) | 247 | 30 | 10.9 (6.8-15.0) | 664 | 53 | 7.0 (5.1-9.0) | |||
Rates for MGUS determined by serum protein electrophoresis, age- and sex-adjusted to the 2000 US total population.
Rates for MGUS determined by either serum protein electrophoresis or immunofixation, age- and sex-adjusted to the 2000 US total population.
Using more sensitive immunofixation methods and the serum FLC assay, the overall frequency of MGUS in all first-degree relatives of either a MM or MGUS proband increased to 9.1%, with an age- and sex-adjusted prevalence of 8.1% (95% CI, 6.3 to 9.8) (Table 2). The age-specific prevalence for ages 40 to 49, 50 to 59, 60 to 69, 70 to 79, and 80 years or older using the sensitive techniques were 1.9%, 6.9%, 11.6%, 14.6%, and 21.0%, respectively. Similar increases in age-specific prevalence were seen when examining relatives of a MM proband (1.0%, 5.7%, 11.9%, 12.1%, 19.4%, respectively; P < .001) or MGUS proband (5.3%, 9.2%, 10.6%, 20.5% and 25.0%, respectively; P < .001) separately.
Comparison of prevalence with reference population
For comparisons with the reference population, only cases detected by protein electrophoresis and confirmed by immunofixation were included so that the diagnostic strategy was identical in the 2 groups being compared. Thus, MGUS detected in first-degree relatives solely on immunofixation without abnormalities on protein electrophoresis was excluded. Using identical strategies to detect MGUS, we found that the age-specific rates in first-degree relatives of MM and MGUS probands combined were significantly higher compared with the Olmsted County reference population (Table 2).
For all first-degree relatives, the risk of MGUS was significantly (P < .001) greater than in the Olmsted County population (risk ratio, 2.6; 95% CI, 1.9 to 3.4). This association was similar across age, sex of relative, age of proband, and relationship of the first-degree relative (Table 3; Figures 1,2). Among first-degree relatives of MM probands only, the age-adjusted rate of MGUS was twice that of the Olmsted County population (risk ratio, 2.0; 95% CI, 1.4 to 2.8). Results were similar when the analyses were restricted to MM probands from southeastern Minnesota (n = 16) (risk ratio, 2.3; 95% CI, 0.75 to 7.21) and MM probands from the state of Minnesota (n = 90) (risk ratio, 2.5; 95% CI, 1.5 to 4.1), populations close in demographics to the reference population. Among first-degree relatives of MGUS probands only, the age-adjusted risk ratio of MGUS was even higher (risk ratio, 3.3; 95% CI, 2.1 to 4.8). However, the difference in risk ratio by proband type (MM or MGUS) did not reach statistical significance (P = .09).
Risk of MGUS by relative and proband characteristics
| Relative or proband characteristic . | All probands (MGUS and MM) . | MGUS probands . | MM probands . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total no. . | Observed/expected MGUS cases . | Risk ratio* . | 95% CI . | P† . | Risk ratio* . | 95% CI . | P† . | Riskratio* . | 95% CI . | P† . | |
| Variables in first-degree relatives | |||||||||||
| Younger than 65 y | 587 | 19/7.75 | 2.45 | (1.55-3.81) | 2.28 | (0.94-5.43) | 2.52 | (1.48-4.21) | |||
| 65 y or older | 324 | 36/15.19 | 2.37 | (1.66-3.18) | .84 | 3.71 | (2.23-5.76) | .37 | 1.79 | (1.11-2.72) | .30 |
| Female | 529 | 28/11.32 | 2.47 | (1.68-3.51) | 3.89 | (2.11-6.89) | 2.00 | (1.22-3.16) | |||
| Male | 382 | 27/11.62 | 2.32 | (1.55-3.29) | .79 | 2.79 | (1.49-4.87) | .42 | 2.08 | (1.24-3.31) | .93 |
| Parent/child | 418 | 17/8.44 | 2.01 | (1.22-3.16) | 2.34 | (0.96-5.57) | 1.90 | (1.05, 3.25) | |||
| Sibling | 493 | 38/14.50 | 2.62 | (1.86-3.52) | .36 | 3.66 | (2.20-5.70) | .40 | 2.13 | (1.36–3.21) | .73 |
| Variables in probands | |||||||||||
| Proband younger than 65 y | 553 | 29/13.51 | 2.15 | (1.46-3.02) | 2.23 | (0.98-4.86) | 2.13 | (1.38-3.12) | |||
| Proband 65 y or older | 358 | 26/9.43 | 2.76 | (1.83-3.94) | .36 | 3.92 | (2.33-6.20) | .25 | 1.87 | (0.98-3.40) | .74 |
| M spike less than 1.5 | 397 | 18/9.92 | 1.81 | (1.12-2.82) | 2.83 | (1.60-4.75) | 0.94 | (0.38-2.21) | |||
| M spike 1.5 or more | 514 | 37/13.02 | 2.84 | (2.01-3.82) | .12 | 4.12 | (2.09-7.70) | .39 | 2.58 | (1.74-3.65) | .04 |
| IgG | 587 | 38/15.0 | 2.53 | (1.80-3.40) | 2.50 | (1.42-4.20) | 2.55 | (1.68-3.68) | |||
| Other‡ | 324 | 17/7.94 | 2.14 | (1.30-3.36) | .56 | 5.68 | (2.87-10.61) | .06 | 1.26 | (0.62-2.46) | .08 |
| Relative or proband characteristic . | All probands (MGUS and MM) . | MGUS probands . | MM probands . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total no. . | Observed/expected MGUS cases . | Risk ratio* . | 95% CI . | P† . | Risk ratio* . | 95% CI . | P† . | Riskratio* . | 95% CI . | P† . | |
| Variables in first-degree relatives | |||||||||||
| Younger than 65 y | 587 | 19/7.75 | 2.45 | (1.55-3.81) | 2.28 | (0.94-5.43) | 2.52 | (1.48-4.21) | |||
| 65 y or older | 324 | 36/15.19 | 2.37 | (1.66-3.18) | .84 | 3.71 | (2.23-5.76) | .37 | 1.79 | (1.11-2.72) | .30 |
| Female | 529 | 28/11.32 | 2.47 | (1.68-3.51) | 3.89 | (2.11-6.89) | 2.00 | (1.22-3.16) | |||
| Male | 382 | 27/11.62 | 2.32 | (1.55-3.29) | .79 | 2.79 | (1.49-4.87) | .42 | 2.08 | (1.24-3.31) | .93 |
| Parent/child | 418 | 17/8.44 | 2.01 | (1.22-3.16) | 2.34 | (0.96-5.57) | 1.90 | (1.05, 3.25) | |||
| Sibling | 493 | 38/14.50 | 2.62 | (1.86-3.52) | .36 | 3.66 | (2.20-5.70) | .40 | 2.13 | (1.36–3.21) | .73 |
| Variables in probands | |||||||||||
| Proband younger than 65 y | 553 | 29/13.51 | 2.15 | (1.46-3.02) | 2.23 | (0.98-4.86) | 2.13 | (1.38-3.12) | |||
| Proband 65 y or older | 358 | 26/9.43 | 2.76 | (1.83-3.94) | .36 | 3.92 | (2.33-6.20) | .25 | 1.87 | (0.98-3.40) | .74 |
| M spike less than 1.5 | 397 | 18/9.92 | 1.81 | (1.12-2.82) | 2.83 | (1.60-4.75) | 0.94 | (0.38-2.21) | |||
| M spike 1.5 or more | 514 | 37/13.02 | 2.84 | (2.01-3.82) | .12 | 4.12 | (2.09-7.70) | .39 | 2.58 | (1.74-3.65) | .04 |
| IgG | 587 | 38/15.0 | 2.53 | (1.80-3.40) | 2.50 | (1.42-4.20) | 2.55 | (1.68-3.68) | |||
| Other‡ | 324 | 17/7.94 | 2.14 | (1.30-3.36) | .56 | 5.68 | (2.87-10.61) | .06 | 1.26 | (0.62-2.46) | .08 |
Compared with reference population.
Comparison of risk ratios between groups for each variable.
Other category consists of IgA, IgM, light chain, and other combined.
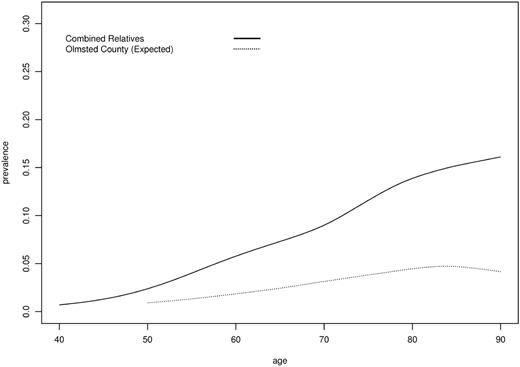
MGUS prevalence by age for all first-degree relatives of MM or MGUS probands compared with Olmsted County reference population. Age > 90 collapsed to 90.
MGUS prevalence by age for all first-degree relatives of MM or MGUS probands compared with Olmsted County reference population. Age > 90 collapsed to 90.
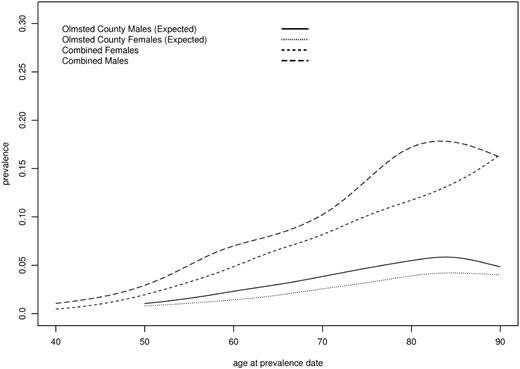
MGUS prevalence by age and sex. All first-degree relatives and Olmsted County reference population. Age > 90 collapsed to 90.
MGUS prevalence by age and sex. All first-degree relatives and Olmsted County reference population. Age > 90 collapsed to 90.
Risk of MGUS in relatives according to M-protein size and immunoglobulin isotype
Because size of the monoclonal protein and immunoglobulin type are predictors of progression to MM and indicative of a high-risk MGUS phenotype, we also evaluated whether the increased risk of MGUS in relatives was specific to probands with a large monoclonal protein concentration or specific monoclonal immunoglobulin isotype. For all MM and MGUS probands, there was a greater risk of MGUS for relatives of probands with high (≥ 1.5 g/dL) M-protein (risk ratio, 2.8; 95% CI, 2.0 to 3.8) compared with lower M-protein levels (risk ratio, 1.8; 95% CI, 1.1 to 2.8), although the difference did not reach statistical significance (P = .12); this increase with high M-protein was seen among relatives of both MGUS and MM probands and was statistically significant among MM probands (Table 3). Among all probands, the risk of MGUS in relatives did not differ by proband's isotype (IgG vs other). The risk ratio was identical (risk ratio, 2.5) for relatives of a MGUS or MM proband with an IgG isotype (Table 3). However, the risk of MGUS in relatives of a proband with a non-IgG isotype differed by whether the proband was MM or MGUS, but the small numbers of non-IgG isotypes limit meaningful interpretation of these estimates.
Characteristics of relative pairs
Table 4 shows the characteristics of MGUS or MM in the 55 relative pairs from 52 families. We found co-occurrence of the monoclonal immunoglobulin isotypes in the same family, but this was similar to the expected distribution of immunoglobulin subtypes in MGUS. In addition, there was no evidence for clustering of M-protein concentration, sex, or age at MGUS diagnosis (Table 4).
Characteristics of MGUS and/or MM in 55 relative pairs*
| Proband characteristic . | . | First-degree relative characteristic . | . | P† . | |
|---|---|---|---|---|---|
| Age, y | <65 | ≥65 | .56 | ||
| Younger than 65 | 9 (31.0) | 20 (69.0) | |||
| 65 or older | 10 (38.5) | 16 (61.5) | |||
| Sex | Female | Male | . | .58 | |
| Female | 14 (56.0) | 11 (44.0) | |||
| Male | 19 (63.3) | 11 (36.7) | |||
| Monoclonal protein concentration, g/dL | <1.5 | ≥1.5 | .25 | ||
| Less than 1.5 | 16 (88.9) | 2 (11.1) | |||
| 1.5 or more | 36 (97.3) | 1 (2.7) | |||
| Monoclonal immunoglobulin isotype | IgG | IgA | IgM | Light chain only | .23 |
| IgG | 32 (84.2) | 2 (5.3) | 2 (5.3) | 2 (5.3) | |
| IgA | 6 (66.7) | 1 (11.1) | 1 (11.1) | 1 (11.1) | |
| IgM | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 (0) | |
| Light chain only | 1 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Proband characteristic . | . | First-degree relative characteristic . | . | P† . | |
|---|---|---|---|---|---|
| Age, y | <65 | ≥65 | .56 | ||
| Younger than 65 | 9 (31.0) | 20 (69.0) | |||
| 65 or older | 10 (38.5) | 16 (61.5) | |||
| Sex | Female | Male | . | .58 | |
| Female | 14 (56.0) | 11 (44.0) | |||
| Male | 19 (63.3) | 11 (36.7) | |||
| Monoclonal protein concentration, g/dL | <1.5 | ≥1.5 | .25 | ||
| Less than 1.5 | 16 (88.9) | 2 (11.1) | |||
| 1.5 or more | 36 (97.3) | 1 (2.7) | |||
| Monoclonal immunoglobulin isotype | IgG | IgA | IgM | Light chain only | .23 |
| IgG | 32 (84.2) | 2 (5.3) | 2 (5.3) | 2 (5.3) | |
| IgA | 6 (66.7) | 1 (11.1) | 1 (11.1) | 1 (11.1) | |
| IgM | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 (0) | |
| Light chain only | 1 (100) | 0 (0) | 0 (0) | 0 (0) | |
All proband and relative pairs in which the relative was positive for MGUS by serum electrophoresis.
P value from chi-squared test.
Discussion
The prevalence of monoclonal gammopathies such as MGUS and MM varies to a striking extent based on race, with rates of MGUS in Japanese and Mexicans less than one-quarter to one-half of those seen in a US Midwestern white population2,6,13 and rates in blacks that are twice as high or greater.3-5 A genetic predisposition to MGUS has been hypothesized to explain racial differences, rather than environmental factors, since a similar increase in risk of MGUS relative to whites was seen both in blacks from Ghana, Africa and blacks in the United States.3,4 A few case reports and case-control studies have suggested a higher risk of MM in first-degree relatives of patients with monoclonal plasma cell disorders, further raising the possibility that there may be a genetic predisposition that affects the incidence of monoclonal gammopathies.8-10,14,15 In addition, reports of multigenerational families with multiple cases of MM and MGUS illustrate the possibility of a shared cancer-susceptibility locus.16,17
In this study, we describe for the first time a clinically and statistically significant increase in the prevalence of MGUS among first-degree relatives of patients with MGUS or MM compared with a reference population, using identical screening and diagnostic techniques. Overall, first-degree family members of a MGUS or MM case have at least a 2-fold greater risk of MGUS compared with population rates. In absolute terms, the prevalence of MGUS (using standard electrophoretic techniques) in first-degree relatives 80 years and older is 13% compared with 7% in the reference population. The increased risk of MGUS was seen across both sexes and all ages of relatives 40 years and older. Our findings provide clear evidence of familial aggregation of MGUS and MM.
The increased risk of MGUS in relatives of MM or MGUS cases implies shared genes and/or environment. There is currently little consistent evidence for environmental risk factors influencing MGUS or MM,18 although some studies suggest positive associations with infectious agents19 and occupational exposures18 and protection from dietary consumption of green vegetables and fish.18 However, in addition to racial differences, several lines of evidence imply a genetic predisposition to MGUS. MGUS is thought to arise following genetic abnormalities (immunoglobulin heavy chain translocations or hyperdiploidy) that arise when a plasma cell divides in response to antigenic stimulation. The constant rate of progression of MGUS to MM (1% per year), which does not increase with duration of MGUS is suggestive of a simple random multihit genetic model of malignancy, in which MGUS is the first event, and progression to MM is the second event. All of the available data so far support that the increased risk of MM seen in blacks compared with whites is due to an increased risk of MGUS rather than a higher risk of progression to MM.3 Thus, it appears that the risk of MGUS, and by extension the risk of genetic abnormalities that establish the premalignant plasma cell clone in MGUS in the first place, may be influenced by baseline inheritable genetic factors. Our findings lend support to this hypothesis that there is an inherent genetic predisposition that affects the occurrence of MGUS.
The implications of our study are important. MGUS is a common clinical condition affecting more than 3% of the general population older than 50 years, and carries a life-long risk of MM or related malignancy. Our finding of an increased risk of MGUS in first-degree relatives adds to the limited number of risk factors that have been identified so far for MGUS, namely age, sex, and race. The results will provide the basis for future studies looking for inherited genetic changes and/or polymorphisms that predispose to the disorder, and improve our understanding of the underlying biology of the condition. Clinically, these data are of great importance when managing care for patients presenting with MGUS. MGUS is often incidentally diagnosed when workup is performed for a possible disease associated with MGUS, such as neuropathy. In patients with MGUS and an associated neuropathy, for example, the association may be causal or coincidental given the high prevalence of both disorders in the general population. In such circumstances, knowledge of an accurate expected baseline rate of MGUS in the given patient can better inform the probability as to whether the association is coincidental or causal in nature and the decision to treat the MGUS.
This is the first prospective study to comprehensively and systematically examine the familial aggregation of MGUS and MM. Other strengths of the study include the use of sensitive assays, age- and gender-adjusted comparison with a well-defined reference population, and the recruitment of a large number of families with both MM and MGUS. We recognize the low response rates for MGUS probands and their relatives, likely due to the benign nature of this condition and advanced age of this population, could have influenced our risk estimates. However, due to the older age of the MGUS probands who were nonrespondents and the increased likelihood of MGUS at advanced age, we would expect their inclusion would only strengthen our findings of familial aggregation. In addition, this study cannot determine whether familial clustering is the result of underlying genetic predisposition or shared environmental factors but provides the basis for further investigation of both.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by the National Cancer Institute, National Institutes of Health, Bethesda, MD (CA62242, CA107476).
National Institutes of Health
Authorship
Contribution: C.M.V. and S.V.R. conceived of the study question and wrote the paper with comments from all coauthors; C.M.V. was responsible for study design and overseeing the project; R.A.K. helped in strategizing the project, contributed study populations, and interpreted study findings; R.A.K. and J.A.K. determined MGUS diagnoses for all relatives; B.J.F. was responsible for participant enrollment, data collection, and human subjects concerns; T.M.T., D.R.L., and C.L.C. performed all statistical analyses and contributed to their interpretation; T.K.P. assisted with data collection and was responsible for all blood processing; A.D. and S.K.K. provided scientific input and interpretation of analyses; all coauthors read and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Celine M. Vachon, Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: vachon.celine@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal