Abstract
In lymphocytes, the second messenger cyclic adenosine monophosphate (cAMP) plays a well-established antiproliferative role through inhibition of G1/S transition and S-phase progression. We have previously demonstrated that, during S-phase arrest, cAMP inhibits the action of S phase–specific cytotoxic compounds, leading to reduction in their apoptotic response. In this report, we provide evidence that cAMP can also inhibit the action of DNA-damaging agents independently of its effect on S phase. Elevation of cAMP in B-cell precursor acute lymphoblastic leukemia cells is shown to profoundly inhibit the apoptotic response to ionizing radiation, anthracyclins, alkylating agents, and platinum compounds. We further demonstrate that this effect depends on the ability of elevated cAMP levels to quench DNA damage–induced p53 accumulation by increasing the p53 turnover, resulting in attenuated Puma and Bax induction, mitochondrial outer membrane depolarization, caspase activation, and poly(ADP-ribose) polymerase cleavage. On the basis of our findings, we suggest that cAMP levels may influence p53 function in malignant cells that retain wild-type p53, potentially affecting p53 both as a tumor suppressor during cancer initiation and maintenance, and as an effector of the apoptotic response to DNA-damaging agents during anticancer treatment.
Introduction
The p53 tumor suppressor is a sequence-specific transcription factor located at the nexus of a complex signaling network, on which signals of various types of cellular stress converge. In humans, the importance of p53 as a tumor suppressor is highlighted by the high frequency of somatic mutations encountered in sporadic cancers,1 as well as by the high incidence and early onset of tumors observed in Li-Fraumeni patients carrying germline mutations of TP53.2 In tumors retaining wild-type p53, its function is thought to be suppressed by viral proteins, deregulation of components of the p53 regulatory circuit, or disruption of upstream or downstream signaling pathways.3
Under normal conditions, p53 is expressed at low levels in the cell because of its rapid degradation. In response to activating signals such as DNA damage, p53 is stabilized and accumulates in the nucleus, where it activates transcription of p53-responsive target genes, leading to phenotypic alterations, including cell-cycle arrest, senescence, or apoptosis. The outcome of p53 activation depends on cell type and the nature and context of DNA damage. For instance, hematopoietic cells typically undergo apoptosis in response to DNA damage, an event that requires the induction of the p53-target gene Puma.4-6 Although activation of p53 constitutes a major barrier to cancer initiation and progression, it is also thought to play an important role in induction of cell death in response to anticancer treatments such as radiation therapy and various chemotherapeutic agents. Indeed, abolition of the p53 function in cancer tissue has been shown to contribute to therapy resistance.7
Cyclic adenosine monophosphate (cAMP) is the prototypical second messenger that is generated by adenylyl cyclase on stimulation of G protein–coupled receptors. In cells of the immune system, cAMP is established as an important signal transducer in several physiologic and pathologic settings.8 For instance, stimulation of the T-cell receptor/CD3 complex results in transient formation of cAMP, limiting further activation of the cells.9,10 In CD4+ T cells from HIV-infected patients, abnormally high levels of cAMP are shown to contribute to the dysfunction of T cells in these persons,11 and in a murine AIDS model, increased cAMP levels and activation of protein kinase A I has been found to contribute to immunodeficiency.12 Lymphocytes possess G protein–coupled receptors for catecholamines and prostaglandin E2, and engagement of these receptors by their respective ligands has been shown to exert a growth-inhibitory effect mediated by the elevation of cAMP levels.13-16 We have previously shown that elevation of intracellular cAMP leads to accumulation of lymphoid cells in G1 phase.17,18 More recently, we demonstrated that cAMP exerts an inhibitory effect on DNA replication, thus leading to arrest of cells in S phase and block of apoptosis induced by S phase–specific anticancer agents.19
Although the importance of cAMP in regulation of lymphocyte activation and proliferation is well established, less is known about its role in cellular response to stress. To examine this, we studied the effect of activation of cAMP signaling on apoptosis induced by various DNA-damaging agents in lymphocytes. We show that elevation of cAMP in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) cells exerts a profound protective effect against DNA damage–induced apoptosis. Furthermore, we show that cAMP inhibits the DNA damage–induced accumulation of p53, an event that appears to be critical for its ability to attenuate apoptosis after a genotoxic insult. Hence, the status of intracellular cAMP in lymphoid cells may be an important determinant in the outcome of treatment regimens that are based on DNA-damaging agents.
Methods
Reagents, antibodies, and radiation treatment
Forskolin, doxorubicin, daunorubicin, 3-isobutyl-1-methylxanthine (IBMX), propidium iodide (PI), paraformaldehyde, saponin, cycloheximide (CHX), phytohemagglutinin (PHA), Staphylococcus aureus Cowan I (SAC), and menadione were from Sigma-Aldrich. MG-132, JC-1, cisplatin, and staurosporine were obtained from Calbiochem. 4-Hydroperoxycyclophosphamide (4-OOH-CP) was from Niomech/IIT. 8-CPT-cAMP was from BioLog. An Annexin V–Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kit was purchased from BD Biosciences Pharmingen, and the In Situ Cell Death Detection Kit was from Roche Applied Sciences. Antibodies were: p53 (DO-1), actin (C2; Santa Cruz Biotechnology); Chk2, Phospho-Chk2 (T68), Bcl-2, Bax, caspase 3, caspase 9, and cleaved poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology); Puma (Serotec); Noxa (Abcam); phospho-ATM (S1981; Rockland); CD19-FITC (Miltenyi Biotec); and CD10-PE-Cy5 (BD Biosciences Pharmingen). For treatment of cells with γ-radiation, cells were irradiated using a 137Cs source at a dose rate of 4.3 Gy/min.
Cell culture and primary cell isolation
The BCP-ALL cell line Reh20 was cultured as previously described.19 TK621 and WTK1 cells22 are both thymidine kinase heterozygous derivatives of the human B lymphoblast cell line WI-L2, isolated from the spleen of a patient with hereditary spherocytosis.23,24 TK6 expresses wild-type p53, whereas WTK1 overexpresses mutant p53 (M237I).25,26 TK6 and WTK1 cells were cultured under similar conditions as Reh cells. EU-3 is a p53 wild-type cell line derived from a pediatric pre-B ALL.27 EU-3 cells were cultured at a density between 1.0 × 106 and 2.0 × 106 in RPMI 1640 as for Reh but with 20% fetal bovine serum and 10 μg/mL ciprofloxacin. For T-lymphocyte isolation, buffy coats from healthy blood donors were purchased from the blood bank at Ullevål University Hospital (Oslo, Norway). Peripheral blood mononuclear cells (PBMCs) were isolated using density-gradient centrifugation with Lymphoprep (Axis-Shield). CD4+ T lymphocytes were then positively selected by use of magnetic CD4 MicroBeads (Miltenyi Biotec). T cells (2.0 × 106 cells/mL) were stimulated with 4 μg/mL PHA, under similar culture conditions as described for Reh cells, for 24 hours before further treatment. B cells were stimulated by treatment of PBMCs with the B-cell polyclonal activator SAC (1:30000) for 24 hours before further treatment. Primary BCP-ALL blasts were isolated from bone marrow aspirates performed at diagnosis from 3 pediatric patients after informed consent by parents, in accordance with the Declaration of Helsinki. The diagnosis of pre-B ALL was later confirmed for all 3 patients. The collection of material has been approved by the Regional Ethics Committee of Norway, region Sør-Øst A, and recommended by Competent Authorities. Mononuclear cells were isolated from the samples by density-gradient centrifugation in Lymphoprep. The proportion of ALL blasts was 74% for ALL 1, 38% for ALL 2, and 81% for ALL 3 as assessed by costaining of cells for CD19 and CD10.
Flow cytometry
For PI staining, cells were incubated with PI (20 μg/mL) at room temperature for 10 minutes before analysis. For cell death analysis in normal peripheral B cells and BCP-ALL blasts, cells were stained with FITC-conjugated CD19 antibodies, and PI uptake was measured in the CD19+ subpopulation. TdT-mediated dUTP nick-end labeling (TUNEL) positivity and externalization of phosphatidyl serine were detected using the In Situ Cell Death Detection Kit and Annexin V–FITC Apoptosis Detection Kit, respectively. Changes in mitochondrial membrane potential (Δψm) were assessed by incubating cells with JC-1 (15 μg/mL) at 37°C for 15 minutes before analysis. Cell death in menadione-treated cells was measured using a forward light scatter and side light scatter gating strategy.28,29
Immunoblotting
For immunoblot analysis, cells were lysed in radioimmunoprecipitation assay buffer, and an equal amount of proteins was separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After transfer to a nitrocellulose membrane (GE Healthcare), proteins were detected by use of standard immunoblotting procedures. For densitometric analysis, blots were scanned and the intensity of protein bands was quantified using the Scion Image software (Scion Corp).
Transfection
Reh cells (6 × 106) were transfected with 10 μg pXJ-E6 plasmid encoding the HPV16 E6 protein or with 16 pmol p53 or control siRNA (sc-29435 or sc-37007, respectively; Santa Cruz Biotechnology) using the Nucleofection solution R and the G-16 program with a Nucleofector device (Amaxa Biosciences). Cells transfected with siRNA or plasmid DNA were incubated for 12 or 24 hours, respectively, after transfection before further treatment. Fluorescence-activated cell sorter (FACS) analysis of Reh cells 24 hours after transfection with pMax-GFP plasmid (Amaxa) revealed a transfection efficiency of 30% to 35%.
Statistical methods and calculation
SPSS 14.0.2 for Windows was used to perform statistical analysis. The paired-samples t test was applied to test significance in cell line experiments, whereas the Wilcoxon signed-rank test was applied to experiments using material from several individual donors. Specific cell death was calculated using the following equation: specific cell death % =(% experimental cell death in the ionizing radiation (IR)–treated or drug-treated sample − % spontaneous cell death in the absence of IR or drug)/(100% − % spontaneous cell death in the absence of IR or drug) × 100. In all figures, histograms show mean values of the indicated number of experiments, with error bars corresponding to SEM values.
Results
cAMP inhibits the IR-induced apoptosis in lymphoid cells
We have previously reported that elevation of intracellular levels of cAMP leads to transient inhibition of DNA replication.19 Moreover, we showed that exposure of cells to S phase-specific antitumor drugs during the cAMP-induced replicative arrest abrogated the apoptotic response of cells to these drugs.19 These data indicated that cAMP is able to inhibit the cytotoxic effect of short-term exposure to S phase–specific DNA-damaging agents through its ability to inhibit the process of DNA replication but did not address whether cAMP can directly affect the cellular response to DNA damage. We therefore initiated the current study to assess the effect of cAMP on DNA-damaging agents whose effects are generally considered to be cell cycle independent.
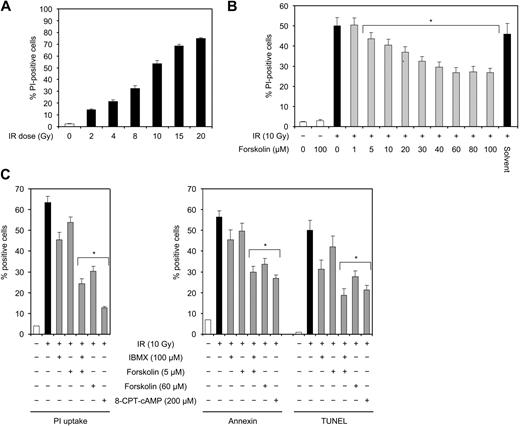
The BCP-ALL cell line Reh was treated with various doses of IR and examined for cell death after 24 hours. As shown in Figure 1A, IR induced cell death in Reh cells in a dose-dependent manner, with approximately 50% cell death after exposure to 10 Gy IR. This dose of IR was used in the following experiments. To examine the effect of cAMP on IR-induced cell death, Reh cells were irradiated in the absence or presence of various concentrations of the cAMP-generating agent forskolin, an activator of adenylyl cyclase.30,31 Figure 1B shows that forskolin inhibited IR-induced cell death in a dose-responsive manner. The IR-induced cell death was maximally reduced by approximately 50% at 60 μM forskolin, and this concentration was chosen for the following experiments.
IR induces dose-dependent cell death in Reh cells that can be inhibited by forskolin. (A) Reh cells were irradiated with the indicated doses of IR and cultured for 24 hours before cell death analysis by PI staining (n = 4). (B) Reh cells were treated with the indicated doses of forskolin or solvent (dimethyl sulfoxide corresponding to 60 μM forskolin) 30 minutes before irradiation with 10 Gy. After 24 hours of incubation, cell death was assessed by PI staining (n = 4). *P < .01 relative to cells treated with IR only. (C) Reh cells were treated with or without IBMX, forskolin, or 8-CPT-cAMP for 90 minutes (IBMX) or 45 minutes (forskolin, 8-CPT-cAMP) before exposure to 10 Gy IR. After 18 hours, cells were analyzed for PI uptake, annexin V binding, and TUNEL positivity by FACS (n = 4). *P < .01 relative to cells treated with IR only.
IR induces dose-dependent cell death in Reh cells that can be inhibited by forskolin. (A) Reh cells were irradiated with the indicated doses of IR and cultured for 24 hours before cell death analysis by PI staining (n = 4). (B) Reh cells were treated with the indicated doses of forskolin or solvent (dimethyl sulfoxide corresponding to 60 μM forskolin) 30 minutes before irradiation with 10 Gy. After 24 hours of incubation, cell death was assessed by PI staining (n = 4). *P < .01 relative to cells treated with IR only. (C) Reh cells were treated with or without IBMX, forskolin, or 8-CPT-cAMP for 90 minutes (IBMX) or 45 minutes (forskolin, 8-CPT-cAMP) before exposure to 10 Gy IR. After 18 hours, cells were analyzed for PI uptake, annexin V binding, and TUNEL positivity by FACS (n = 4). *P < .01 relative to cells treated with IR only.
Forskolin, in addition to its ability to raise the intracellular levels of cAMP through direct stimulation of adenylyl cyclase, has also been shown to influence certain cellular processes independent of cAMP.32,33 To assess whether the inhibitory effect of forskolin on IR-induced cell death is mediated by cAMP, Reh cells were irradiated in the absence or presence of IBMX or 8-CPT-cAMP. IBMX inhibits the phosphodiesterase-mediated degradation of cAMP to AMP, resulting in increased cAMP levels within the cell. 8-CPT-cAMP is a membrane-permeable analog of cAMP. As measured by PI uptake, exposure of cells to 5 μM forskolin or 100 μM IBMX alone led to slight inhibition of IR-induced cell death (Figure 1C left panel). However, cotreatment of cells with forskolin and IBMX, which augments cAMP accumulation by activating adenylyl cyclase and blocking phosphodiesterases, respectively, inhibited IR-induced cell death to a level comparable with that induced by 60 μM forskolin. Similarly, exposure of cells to 8-CPT-cAMP substantially decreased cell death induced by IR (Figure 1C left panel). These results show that cAMP has an inhibitory effect on IR-induced cell death.
To address whether the cell death induced by IR in Reh cells bears the typical hallmarks of apoptosis, Reh cells shown in Figure 1C (left panel) were also analyzed for phosphatidyl serine exposure by annexin-V staining, and DNA fragmentation by TUNEL staining. As shown in Figure 1C (right panel), treatment of Reh cells with 10 Gy IR led to the appearance of essentially similar levels of annexin V–labeled and TUNEL-positive cells, both of which were comparable with the level of IR-induced PI-positive cells. Moreover, cotreatment of cells with cAMP-elevating agents or 8-CPT-cAMP reduced the appearance of annexin V–stained and TUNEL-positive cells. Thus, cell death induced by treatment of Reh cells with IR is essentially apoptotic.
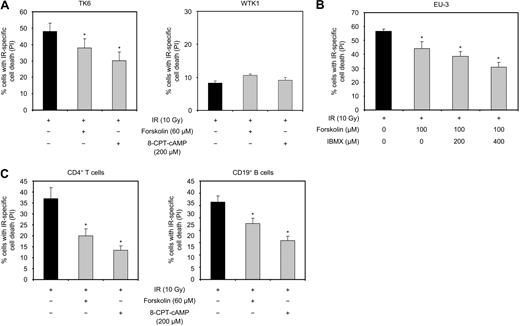
To examine whether the inhibition of IR-induced apoptosis represents a general action of cAMP, we examined the effect of cAMP on IR-induced apoptosis in the lymphoid cell lines TK6 and EU-3, as well as in normal human CD4+ T cells and CD19+ B cells. TK6 cells22 are derived from the human B lymphoblast cell line WI-L2,23 and EU-3 cells have been derived from a pediatric pre-B ALL.27 As shown in Figure 2A, exposure of TK6 cells to 10 Gy IR in the presence of forskolin or 8-CPT-cAMP led to reduction in IR-induced cell death. Pretreatment of EU-3 cells with 60 μM forskolin had only marginal inhibitory effect on cell death induced by IR (data not shown). However, exposure of the cells to 100 μM forskolin was found to decrease the IR-mediated cell death (Figure 2B). This effect of forskolin was augmented by cotreatment of cells with IBMX, suggesting that EU-3 cells are less responsive to the cAMP-generating effect of forskolin than Reh or TK6. Finally, forskolin and 8-CPT-cAMP were also found to inhibit IR-induced apoptosis in normal human CD4+ T cells and CD19+ B cells (Figure 2C). Taken together, these results demonstrate that cAMP has an inhibitory effect on cell death induced by IR in a variety of transformed and nontransformed human cell types of lymphoid origin.
cAMP inhibits IR-induced cell death in different lymphoid cell types. (A) TK6 and WTK1 cells were cultured in the absence or presence of forskolin or 8-CPT-cAMP for 30 minutes before exposure to 10 Gy IR. After 18 hours, cells were analyzed for PI uptake (n = 4). *P < .05 (relative to cells treated with IR only). (B) EU-3 cells were treated with or without IBMX or forskolin for 90 minutes or 30 minutes, respectively, before 10 Gy IR. Cells were analyzed for PI uptake 12 hours after IR (n = 4). *P < .05 (relative to cells treated with IR only). (C) PHA-stimulated human CD4+ T cells or SAC-stimulated PBMCs were treated with or without forskolin or 8-CPT-cAMP for 30 minutes before exposure to 10 Gy IR. After 24 hours, cells were analyzed for PI uptake. The B-cell fraction of PBMCs was identified by staining the cells with FITC-conjugated antibodies against CD19, and PI uptake was assessed in this subpopulation (T cells, n = 5; B cells, n = 6). *P < .05 relative to cells treated with IR only.
cAMP inhibits IR-induced cell death in different lymphoid cell types. (A) TK6 and WTK1 cells were cultured in the absence or presence of forskolin or 8-CPT-cAMP for 30 minutes before exposure to 10 Gy IR. After 18 hours, cells were analyzed for PI uptake (n = 4). *P < .05 (relative to cells treated with IR only). (B) EU-3 cells were treated with or without IBMX or forskolin for 90 minutes or 30 minutes, respectively, before 10 Gy IR. Cells were analyzed for PI uptake 12 hours after IR (n = 4). *P < .05 (relative to cells treated with IR only). (C) PHA-stimulated human CD4+ T cells or SAC-stimulated PBMCs were treated with or without forskolin or 8-CPT-cAMP for 30 minutes before exposure to 10 Gy IR. After 24 hours, cells were analyzed for PI uptake. The B-cell fraction of PBMCs was identified by staining the cells with FITC-conjugated antibodies against CD19, and PI uptake was assessed in this subpopulation (T cells, n = 5; B cells, n = 6). *P < .05 relative to cells treated with IR only.
cAMP inhibits apoptosis induced by a variety of DNA-damaging cytotoxic agents
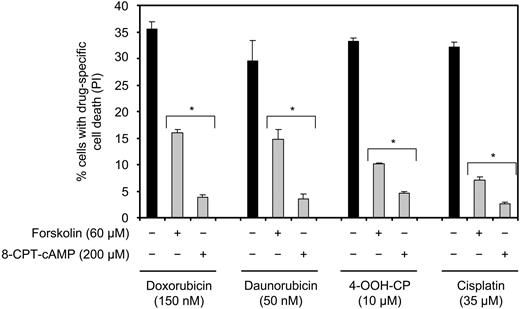
Because of the systemic nature of the majority of malignancies arising from the lymphoid cell compartment, combinatorial chemotherapeutic regimens constitute the treatment of choice. Radiation therapy is normally reserved for localized palliative treatment, with the exception of Hodgkin lymphoma and early-stage non-Hodgkin lymphoma, in which it is used with curative intent. Therefore, we wished to examine whether cAMP also regulates cell death induced by DNA-damaging agents other than IR. To this end, Reh cells were treated with doxorubicin, daunorubicin, 4-OOH-CP, or cisplatin in the presence or absence of forskolin or 8-CPT-cAMP, and examined for cell death after 18 hours. Doxorubicin and daunorubicin are anthracyclins, and 4-OOH-CP is an active metabolite of the alkylating agent cyclophosphamide, all of which are commonly used in the treatment of ALL and intermediate to high-grade non-Hodgkin lymphoma. Cisplatin is a DNA cross-linking agent that is widely used in the treatment of testicular, ovarian, or lung cancer. All these drugs are known to cause DNA damage in a cell cycle–nonspecific manner. As shown in Figure 3, treatment of Reh cells with doxorubicin, daunorubicin, 4-OOH-CP, or cisplatin alone led to induction of cell death. Cotreatment of cells with forskolin or 8-CPT-cAMP led to substantial reduction in cell death induced by all 4 agents. Collectively, these results show that cAMP inhibits cell death induced by a broad spectrum of DNA-damaging therapeutic agents.
cAMP inhibits cell death induced by DNA-damaging chemotherapeutic drugs. Reh cells were treated with or without forskolin or 8-CPT-cAMP for 30 minutes before addition of doxorubicin, daunorubicin, 4-OOH-CP, or cisplatin. Cells were then cultured for 18 hours before PI staining and FACS analysis (n = 4). *P < .01 compared with the corresponding samples treated with chemotherapeutics alone.
cAMP inhibits cell death induced by DNA-damaging chemotherapeutic drugs. Reh cells were treated with or without forskolin or 8-CPT-cAMP for 30 minutes before addition of doxorubicin, daunorubicin, 4-OOH-CP, or cisplatin. Cells were then cultured for 18 hours before PI staining and FACS analysis (n = 4). *P < .01 compared with the corresponding samples treated with chemotherapeutics alone.
Enhanced levels of cAMP attenuate p53 accumulation independent of ATM and Chk2 kinases
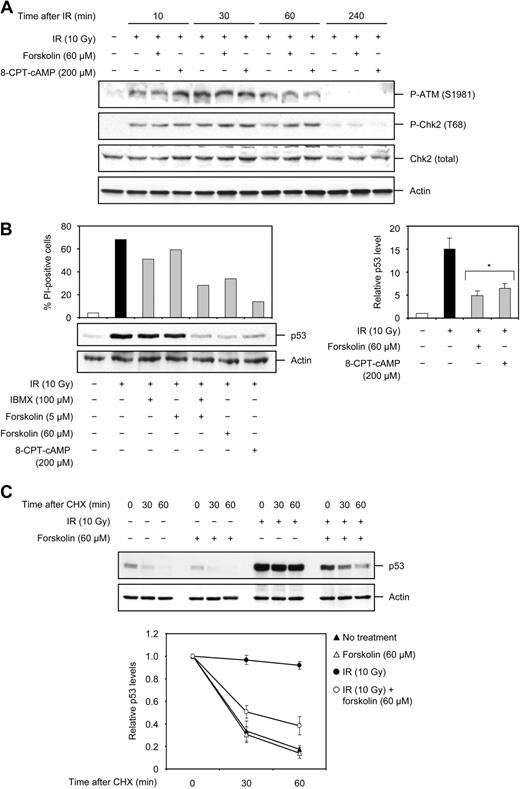
It is known that IR-induced DNA damage initiates signaling through the ATM and Chk2 kinases, whose activities contribute to activation of the p53 tumor suppressor protein. In turn, p53 regulates several transcriptional and nontranscriptional programs that ultimately culminate in a cellular response such as apoptosis. To examine the level at which cAMP interacts with the IR-induced signaling pathway to inhibit apoptosis, Reh cells were first exposed to 10 Gy IR in the absence or presence of forskolin or 8-CPT-cAMP and examined for activation of ATM and Chk2 kinases using phosphospecific antibodies. Consistent with previous reports, ATM was rapidly activated after IR treatment, as assessed by its phosphorylation at S1981 (Figure 4A). Phosphorylation of ATM at S1981 was readily detectable after 10 minutes, reaching its maximum within 30 minutes, and declining thereafter to control levels by 4 hours after IR. Similar to ATM, activation of Chk2, monitored by its phosphorylation at T68, was observed at 10 minutes after IR and then returned to control levels by 4 hours after IR (Figure 4A). Forskolin or 8-CPT-cAMP did not prevent the IR-induced activation of ATM and Chk2, suggesting that these proteins do not contribute to cAMP-mediated inhibition of IR-induced apoptosis.
cAMP does not affect the IR-induced activation of ATM or Chk2 but attenuates p53 accumulation by decreasing its stability. (A) Reh cells were treated with or without forskolin or 8-CPT-cAMP for 30 minutes before exposure to 10 Gy IR. Cells were harvested at the indicated times after IR and subjected to Western blot analysis with the indicated antibodies. The figure shows one representative blot of 3 experiments. (B) Reh cells were treated as described in the legend to Figure 1C. Four hours after IR treatment, a portion of cells was harvested and examined for the expression of p53 and actin by immunoblotting. The histogram shows the level of cell death, measured by analysis of PI uptake, in the remaining portion of cells 18 hours after IR treatment. The right panel shows the densitometric analysis of the p53 band with the value obtained for untreated cells set as 1 (n = 5). *P < .01 (relative to cells treated with IR only). (C) Reh cells were treated with or without forskolin for 30 minutes before exposure to 10 Gy IR. After 4 hours, cells were exposed to CHX (25 μg/mL) over a 60-minute time course. Cells were harvested at the indicated time points after addition of CHX and analyzed for the expression of p53 and actin proteins by immunoblotting. The immunoblot is representative of 4 independent experiments. (Bottom panel) The immunoblots were scanned and the p53 band intensity was normalized to that of actin. The obtained values were then plotted with the value for cells not treated with CHX set as 1 (n = 4).
cAMP does not affect the IR-induced activation of ATM or Chk2 but attenuates p53 accumulation by decreasing its stability. (A) Reh cells were treated with or without forskolin or 8-CPT-cAMP for 30 minutes before exposure to 10 Gy IR. Cells were harvested at the indicated times after IR and subjected to Western blot analysis with the indicated antibodies. The figure shows one representative blot of 3 experiments. (B) Reh cells were treated as described in the legend to Figure 1C. Four hours after IR treatment, a portion of cells was harvested and examined for the expression of p53 and actin by immunoblotting. The histogram shows the level of cell death, measured by analysis of PI uptake, in the remaining portion of cells 18 hours after IR treatment. The right panel shows the densitometric analysis of the p53 band with the value obtained for untreated cells set as 1 (n = 5). *P < .01 (relative to cells treated with IR only). (C) Reh cells were treated with or without forskolin for 30 minutes before exposure to 10 Gy IR. After 4 hours, cells were exposed to CHX (25 μg/mL) over a 60-minute time course. Cells were harvested at the indicated time points after addition of CHX and analyzed for the expression of p53 and actin proteins by immunoblotting. The immunoblot is representative of 4 independent experiments. (Bottom panel) The immunoblots were scanned and the p53 band intensity was normalized to that of actin. The obtained values were then plotted with the value for cells not treated with CHX set as 1 (n = 4).
We next examined the effect of cAMP on p53 accumulation in Reh cells, and as shown in Figure 4B (left panel), exposure of Reh cells to 10 Gy IR led to substantial accumulation of p53 within 4 hours. Treatment of cells with 5 μM forskolin or IBMX alone slightly prevented the IR-mediated induction of p53 levels, whereas exposure of cells to a combination of 5μM forskolin and IBMX, or 60 μM forskolin, or 8-CPT-cAMP greatly prevented the IR-induced accumulation of p53. It is noteworthy that the inhibition of IR-induced p53 accumulation seen with cAMP-elevating agents correlates with their ability to inhibit IR-induced apoptosis. Quantification of the p53 band revealed a 15-fold increase in the level of p53 within 4 hours after IR (Figure 4B right panel). In the presence of forskolin or 8-CPT-cAMP, this increase was reduced to approximately 5-fold. These data clearly show that cAMP exerts a marked inhibitory effect on IR-induced p53 accumulation, independent of ATM and Chk2 activities.
Accumulation of p53 in response to DNA damage is intimately related to its stabilization. Therefore, the observation that cAMP abrogates the IR-induced accumulation of p53 prompted us to investigate whether cAMP affects the stability of p53. To this end, Reh cells treated with IR in the absence or presence of forskolin were exposed to the protein synthesis inhibitor CHX. Cell lysates were then prepared at different times after CHX treatment and analyzed for the level of p53 by immunoblotting. In agreement with previous reports, whereas p53 exhibited a short half-life (< 30 minutes) in unstressed cells, it was substantially stabilized in response to IR treatment (Figure 4C). Interestingly, forskolin profoundly decreased the half-life of p53 protein in IR-treated cells (to ∼ 30 minutes). These results indicate that cAMP antagonizes the IR-induced accumulation of p53 by reducing the stability of p53 protein.
The stability of p53 is primarily regulated by the Mdm2 protein that binds to p53 and triggers its degradation.34 In response to DNA damage, p53 is phosphorylated at various sites in its N-terminus. In particular, phosphorylations at S15, T18, and S20 residues have been suggested to inhibit the interaction of p53 with Mdm2 and thus lead to stabilization of p53. Therefore, to gain insight into the mechanism by which cAMP inhibits the DNA damage–induced stabilization of p53, we wished to examine the effect of forskolin on Mdm2 levels as well as the phosphorylation status of p53 after exposure of cells to IR. To do so, Reh cells were exposed to 10 Gy IR in the absence or presence of 60 μM forskolin and harvested at 4 hours after IR. Cell lysates were then examined for the levels of Mdm2 protein as well as the phosphorylation pf p53 on S15, T18, and S20 residues. Forskolin was found to have no significant effect on the level of Mdm2 protein (data not shown), indicating that the inhibitory effect of cAMP on p53 stability occurs in the absence of an increase in Mdm2 levels. Furthermore, whereas forskolin had no significant effect on the IR-induced phosphorylation of p53 at S15 or T18 residues, it reduced the IR-mediated phosphorylation of p53 at S20 (data not shown). Because the phosphorylation of T18 is the only modification found to abrogate the interaction of p53 with Mdm2, thereby affecting the stability of p53 in the cell,35-37 we cannot at this point conclude that the cAMP-induced destabilization of p53 is mediated through inhibition of the IR-induced phosphorylation of p53 at S20.
cAMP inhibits the IR-induced induction of Puma and Bax, mitochondrial membrane depolarization, caspase activation, and PARP cleavage
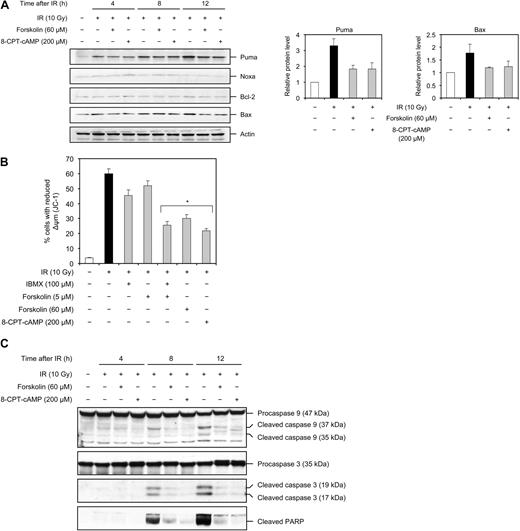
p53 is shown to regulate apoptosis mainly through its ability to transcriptionally up-regulate a variety of proapoptotic proteins, such as Puma, Noxa, and Bax, and/or down-regulate the expression of antiapoptotic proteins, such as Bcl-2. In particular, Puma has been shown to be indispensable for p53-dependent apoptosis in many cells of the lymphoid compartment.4-6,38 Given the ability of cAMP to attenuate the IR-induced accumulation of p53, it was therefore important to determine whether cAMP affected the expression of these proteins. As shown in Figure 5A (left panel), Puma was induced within 4 hours after treatment of Reh cells with 10 Gy of IR and increased progressively by 12 hours after IR treatment. The levels of Bax were also induced by IR, albeit to a lesser extent compared with Puma, whereas the levels of Noxa and Bcl-2 proteins remained unaffected. Importantly, treatment of cells with forskolin or 8-CPT-cAMP had an inhibitory effect on IR-mediated induction of Puma and Bax proteins (Figure 5A). In sum, these results establish a correlation between the inhibitory effect of cAMP on IR-induced accumulation of p53 and IR-mediated induction of Puma and Bax proteins and suggest that cAMP exerts a negative effect on IR-induced activation of p53 as a transcription factor.
cAMP inhibits the IR-induced Puma and Bax expression, mitochondrial membrane depolarization, and cleavage of caspase 9, caspase 3, and PARP. (A) Reh cells were treated as described in the legend to Figure 4A, harvested at the indicated times, and analyzed by immunoblotting with the indicated antibodies. The figure shows 1 representative experiment of 3. (Right panel) The densitometric analysis of the Puma and Bax bands at 12 hours after IR with the value obtained for untreated cells set as 1 (n = 3). (B) Reh cells were treated as described in the legend to Figure 1C. Eighteen hours after IR, cells were examined for Δψm using JC-1 (n = 4). *P < .01 relative to cells treated with IR only. (C) Reh cells were treated as described in the legend to Figure 4A, harvested at the indicated times after IR, and examined by immunoblotting with the indicated antibodies. The figure shows 1 representative experiment of 3.
cAMP inhibits the IR-induced Puma and Bax expression, mitochondrial membrane depolarization, and cleavage of caspase 9, caspase 3, and PARP. (A) Reh cells were treated as described in the legend to Figure 4A, harvested at the indicated times, and analyzed by immunoblotting with the indicated antibodies. The figure shows 1 representative experiment of 3. (Right panel) The densitometric analysis of the Puma and Bax bands at 12 hours after IR with the value obtained for untreated cells set as 1 (n = 3). (B) Reh cells were treated as described in the legend to Figure 1C. Eighteen hours after IR, cells were examined for Δψm using JC-1 (n = 4). *P < .01 relative to cells treated with IR only. (C) Reh cells were treated as described in the legend to Figure 4A, harvested at the indicated times after IR, and examined by immunoblotting with the indicated antibodies. The figure shows 1 representative experiment of 3.
Puma belongs to the BH3-only subgroup of Bcl-2 family proteins, coupling diverse stress signals, including IR, to activation of the intrinsic apoptotic pathway. In this capacity, the BH3-only proteins bind to and inhibit antiapoptotic members, such as Bcl-2. This leads to oligomerization of Bax and/or Bak proteins and their insertion into the mitochondrial outer membrane, resulting in mitochondrial outer membrane permeabilization (MOMP).39 Given the observation that cAMP-mediated inhibition of apoptosis induced by IR was associated with its ability to attenuate the IR-induced induction of Puma and Bax, we considered it important to confirm that cAMP exerts an inhibitory effect on these downstream events. To this end, Reh cells were treated with 10 Gy IR in the absence or presence of forskolin or IBMX alone, the combination of the two, or 8-CPT-cAMP and examined for MOMP, by monitoring the mitochondrial membrane potential (Δψm). As shown in Figure 5B, IR reduced the Δψm in approximately 60% of Reh cells 18 hours after treatment. Low concentrations of forskolin (5 μM) or IBMX alone slightly decreased the number of cells with reduced Δψm. However, a combination of the two substantially inhibited the IR-induced reduction in Δψm. Similarly, treatment of cells with 60 μM forskolin or 8-CPT-cAMP significantly attenuated the decrease in Δψm by IR.
MOMP allows for the release of cyctochrome c from the mitochondrial intermembrane space into cytosol where it contributes in the formation of the apoptosome, which mediates autocleavage and activation of caspase 9. Once activated, caspase 9 cleaves and activates various effector caspases, including caspase 3, which in turn carries out the proteolysis of several substrates, such as PARP, during the execution phase of apoptosis.39 We next examined the effect of forskolin or 8-CPT-cAMP on IR-induced cleavage of caspase 9, caspase 3, and PARP. Reh cells were irradiated in the absence or presence of forskolin or 8-CPT-cAMP and examined for the expression of cleavage products of caspase 9, caspase 3, and PARP by Western blot analysis. As seen in Figure 5C, cleaved fragments of caspase 9, caspase 3, and PARP were detected by 8 hours and increased further by 12 hours after IR. As expected, treatment of cells with forskolin or 8-CPT-cAMP prevented the IR-induced processing of all 3 proteins. These data show that, in accordance with its inhibitory effect on IR-induced apoptosis, cAMP prevents the IR-mediated activation of the proteins known to be involved in execution of apoptosis.
p53 is required for cAMP-mediated inhibition of cell death induced by IR
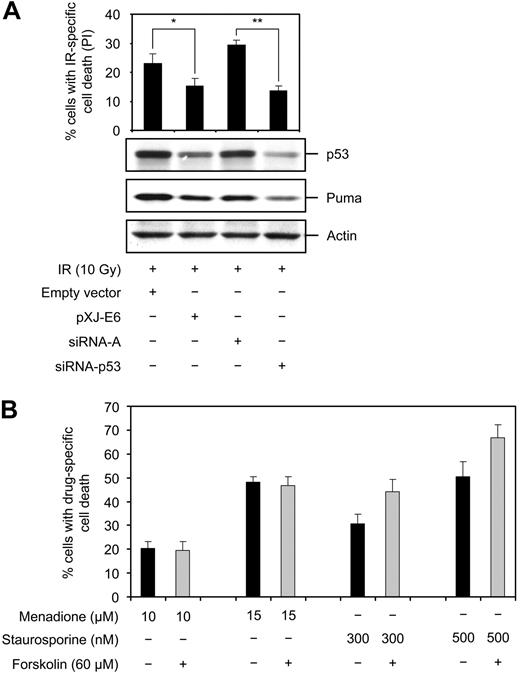
So far we have presented data demonstrating that cAMP interferes with the IR-induced signaling pathway at the level of p53, suggesting that the inhibitory effect of cAMP on IR-induced apoptosis is dependent on p53. To provide evidence for this hypothesis, we aimed to examine the effect of cAMP on IR-mediated apoptosis in the absence of p53. To this end, we transfected Reh cells with siRNA against p53 or the pXJ-E6 plasmid expressing the HPV16 E6 protein, and examined them for the expression of p53 and cell death after irradiation. Western blot anlysis revealed that transfection of Reh cells with p53 siRNA or the pXJ-E6 plasmid inhibited, albeit not completely, the IR-induced accumulation of p53 (Figure 6A). Furthermore, in accordance with a role of Puma as p53 target in IR-induced apoptotic cascade, the IR-mediated induction of Puma was also reduced in cells in which the accumulation of p53 was impeded. Notwithstanding our inability to completely abrogate the IR-induced accumulation of p53, there was a correlation between reduction of p53 levels and reduction of cell death in irradiated cells, supporting the notion that IR-induced cell death in Reh cells is dependent on p53.
p53 is required for the inhibitory effect of cAMP on IR-induced apoptosis. (A) Reh cells were transfected with the pXJ-E6 plasmid expressing the HPV16-strain E6-protein, empty vector, siRNA-p53, or control siRNA-A. Cells were then exposed to 10 Gy IR or left untreated. After 12 hours, a portion of the cells was stained with PI for cell death analysis, and the remaining portion was subjected to immunoblotting with the indicated antibodies (n = 3). *P < .05; **P < .01. (B) Reh cells were incubated with or without forskolin for 30 minutes before addition of menadione or staurosporine, and examined for PI uptake after 18 hours. Because menadione interferes with PI fluorescence, the shift in scatter profile was used to discriminate between dead and viable menadione-treated cells (menadione, n = 3; staurosporine, n = 4).
p53 is required for the inhibitory effect of cAMP on IR-induced apoptosis. (A) Reh cells were transfected with the pXJ-E6 plasmid expressing the HPV16-strain E6-protein, empty vector, siRNA-p53, or control siRNA-A. Cells were then exposed to 10 Gy IR or left untreated. After 12 hours, a portion of the cells was stained with PI for cell death analysis, and the remaining portion was subjected to immunoblotting with the indicated antibodies (n = 3). *P < .05; **P < .01. (B) Reh cells were incubated with or without forskolin for 30 minutes before addition of menadione or staurosporine, and examined for PI uptake after 18 hours. Because menadione interferes with PI fluorescence, the shift in scatter profile was used to discriminate between dead and viable menadione-treated cells (menadione, n = 3; staurosporine, n = 4).
To circumvent the problem with incomplete abrogation of p53 expression in Reh cells, we examined the effect of forskolin or 8-CPT-cAMP on IR-induced cell death in the WTK1 cell line. These cells are the p53 mutated (overexpressing p53 M237I) isogenic variant of TK6 cells. Similar to Reh, the IR-induced cell death in TK6 cells is inhibited by cAMP (Figure 2A left panel). As shown in Figure 2A (right panel), 10 Gy IR-induced cell death in WTK1 cells, but significantly less efficiently compared with TK6 cells. Furthermore, forskolin or 8-CPT-cAMP had no inhibitory effect on IR-induced cell death in WTK1 cells.
Finally, the requirement of p53 for the apoptosis-inhibitory effect of cAMP was verified by examining the effect of forskolin or 8-CPT-cAMP on cell death induced by p53-independent cytotoxic insults, such as staurosporine4,40,41 and menadione.42,43 Staurosporine is a broad-spectrum inhibitor of protein kinases, and menadione is an inducer of reactive oxygen species. Both staurosporine and menadione induced cell death in a dose-dependent manner in Reh cells (Figure 6B). Importantly, cotreatment of cells with forskolin or 8-CPT-cAMP had no inhibitory effect on cell death induced by these 2 agents. In sum, the observations presented in Figures 2 and 6 show that (1) the IR-induced cell death in Reh cells appears to depend on p53 and (2) cAMP depends on p53 to exert its apoptosis-inhibitory effect.
cAMP inhibits DNA damage–induced p53 accumulation and cell death in primary BCP-ALL cells
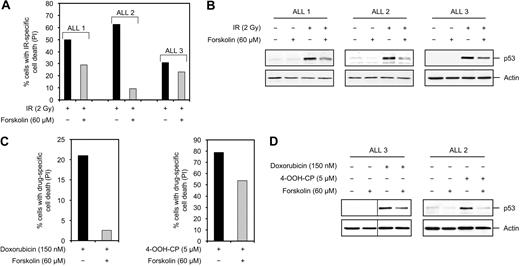
To further confirm the significance of the inhibitory effect of cAMP on DNA damage–induced cell death in ALLs, we used primary BCP-ALL cells isolated from bone marrow aspirates of 3 patients with pediatric BCP-ALL (hereafter referred to as ALL 1, ALL 2, and ALL 3; patient characteristics are outlined in Table 1). Exposure of these cells to various doses of IR revealed that 2 Gy IR induced the maximum level of cell death, as measured by PI uptake, by 18 hours after treatment (data not shown). Importantly, exposure of cells to 2 Gy IR in the presence of 60 μM forskolin led to suppression of IR-induced cell death (Figure 7A). To assess whether cAMP negatively regulates the IR-induced accumulation of p53 in primary BCP-ALL cells, the lysates from the cells in Figure 7A were examined for the level of p53. As shown in Figure 7B, 2 Gy IR led to accumulation of p53 in all 3 ALL samples within 4 hours. Cotreatment of cells with 60 μM forskolin was found to inhibit the DNA damage–mediated induction of p53 in these cells. As mentioned previously, the majority of lymphoid malignancies are treated with combinatorial chemotherapeutic regimens rather than radiotherapy. The exclude the possibility that the inhibitory effect of cAMP is limited to IR-induced cell death, ALL 1 cells were treated with doxorubicin or 4-OOH-CP in the presence or absence of forskolin, and examined for cell death after 18 hours. As shown in Figure 7C, treatment of ALL 1 cells with doxorubicin or 4-OOH-CP alone led to induction of cell death, whereas cotreatment of cells with forskolin inhibited cell death induced by these agents. Finally, we wished to assess whether cAMP regulates the expression of p53 protein in response to doxorubicin or 4-OOH-CP. To do so, ALL 3 and ALL 2 cells were treated with doxorubicin or 4-OOH-CP, respectively, in the absence or presence of forskolin and examined for expression of p53 after 4 hours. Figure 7D shows that treatment of cells with doxorubicin or 4-OOH-CP led to accumulation of p53 protein in these cells. However, cotreatment of cells with forskolin inhibited the doxorubicin- or 4-OOH-CP–induced accumulation of p53. These results indicate that, similar to ALL cell lines, cAMP exerts an inhibitory effect on DNA damage–mediated accumulation of p53 and cell death in primary BCP-ALL cells.
Patient characteristics
| . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Age, y | 2.9 | 1.8 | 1.8 |
| Sex | Female | Female | Male |
| Bone marrow infiltration at diagnosis | 74% | 38% | 81% |
| Cytogenetics | t(12;21)(p13;q22) | Normal karyotype | Normal karyotype |
| Residual disease at day 15 | 2 × 10−4 | 17.3 × 10−2 | 2 × 10−3 |
| Residual disease at day 29 | No MRD | 4.2 × 10−3 | No MRD |
| . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Age, y | 2.9 | 1.8 | 1.8 |
| Sex | Female | Female | Male |
| Bone marrow infiltration at diagnosis | 74% | 38% | 81% |
| Cytogenetics | t(12;21)(p13;q22) | Normal karyotype | Normal karyotype |
| Residual disease at day 15 | 2 × 10−4 | 17.3 × 10−2 | 2 × 10−3 |
| Residual disease at day 29 | No MRD | 4.2 × 10−3 | No MRD |
MRD indicates minimal residual disease.
Suppression of DNA damage–induced p53 accumulation and cell death in primary BCP-ALL cells by cAMP. (A) BCP-ALL cells isolated from bone marrow aspirates of 3 patients with pediatric BCP-ALL (ALL 1, ALL 2, and ALL 3) were treated with or without forskolin for 30 minutes before irradiation with 2 Gy. After 18 hours of incubation, cell death was assessed by costaining the cells with FITC-conjugated anti-CD19 antibodies and PI and analyzing PI uptake in the CD19+ subpopulation. (B) A portion of ALL cells in panel A were harvested 4 hours after IR treatment and subjected to Western blot analysis with p53 and actin antibodies. (C) ALL 1 cells were treated with or without forskolin for 30 minutes before addition of doxorubicin or 4-OOH-CP. Cells were then cultured for 18 hours before costaining with FITC-conjugated anti-CD19 antibodies and PI followed by FACS analysis as described in panel A. (D) ALL 3 and ALL 2 cells were treated with or without forskolin for 30 minutes before addition of doxorubicin or 4-OOH-CP, respectively. After 4 hours, cells were harvested and subjected to Western blot analysis with the indicated antibodies. Vertical lines have been inserted to indicate repositioned gel lanes.
Suppression of DNA damage–induced p53 accumulation and cell death in primary BCP-ALL cells by cAMP. (A) BCP-ALL cells isolated from bone marrow aspirates of 3 patients with pediatric BCP-ALL (ALL 1, ALL 2, and ALL 3) were treated with or without forskolin for 30 minutes before irradiation with 2 Gy. After 18 hours of incubation, cell death was assessed by costaining the cells with FITC-conjugated anti-CD19 antibodies and PI and analyzing PI uptake in the CD19+ subpopulation. (B) A portion of ALL cells in panel A were harvested 4 hours after IR treatment and subjected to Western blot analysis with p53 and actin antibodies. (C) ALL 1 cells were treated with or without forskolin for 30 minutes before addition of doxorubicin or 4-OOH-CP. Cells were then cultured for 18 hours before costaining with FITC-conjugated anti-CD19 antibodies and PI followed by FACS analysis as described in panel A. (D) ALL 3 and ALL 2 cells were treated with or without forskolin for 30 minutes before addition of doxorubicin or 4-OOH-CP, respectively. After 4 hours, cells were harvested and subjected to Western blot analysis with the indicated antibodies. Vertical lines have been inserted to indicate repositioned gel lanes.
Discussion
In this study, we provide evidence that cAMP exerts an inhibitory effect on DNA damage–induced apoptosis in primary BCP-ALL cells and cell lines as well as normal lymphoid cells. Furthermore, we show that this inhibitory effect of cAMP requires p53 and correlates with its ability to abrogate DNA damage–induced accumulation of p53.
Activation of p53-dependent growth arrest or apoptosis is known to constitute an important barrier to cancer initiation, maintenance, and progression.44-46 Not surprisingly, normal function of p53 has been found to be abrogated by its mutational inactivation in approximately one-half of human cancers. In most of the remaining cases, such as the majority of malignancies that originate from the hematopoietic compartment, the function of wild-type p53 is assumed to be antagonized through indirect mechanisms.3 Thus, releasing the wild-type p53 from indirect suppression would be crucial to successful killing of these tumor cells by DNA-damaging therapeutic agents. Our finding that cAMP exerts a potent inhibition of DNA damage–induced accumulation of p53 and apoptosis raises the possibility that aberrant activation of the cAMP signaling pathway may indeed be a mechanism that is used by cancer cells to antagonize the function of p53. Furthermore, our results may have implications for cancer treatment, predicting that neutralization of cAMP signaling in malignant cells with high cAMP levels would render them more sensitive to apoptosis induced by DNA-damaging therapeutic agents.
Several studies have reported that the cAMP signaling pathway may protect against or induce cell death in a manner that appears to depend on the cell type as well as the nature of the death-inducing signal. However, data on the effect of cAMP on DNA damage–induced apoptosis are scarce. The apoptosis-inhibitory effect of cAMP presented here is in agreement with a study by Garcia-Bermejo et al reporting that cAMP decreases DNA damage–induced apoptosis in U-937 promonocytic cells.47 In contrast, an analog of cAMP, 8-Cl-cAMP, was reported to potentiate IR-induced apoptosis in prostate cancer cells,48 underlining the cell-type specificity of cAMP signaling.
In the majority of lymphoid cells, induction of p53 protein is necessary for efficient killing of cells by DNA-damaging agents.49 Consequently, its absence confers pronounced resistance to DNA damage–induced apoptosis. Our data demonstrating that cAMP abrogates the IR-induced accumulation of p53 provide an explanation for how cAMP, at least in the face of genotoxic stress, can inhibit apoptosis. This notion is strengthened by our finding that IR-induced apoptosis in lymphoid cells is refractory to cAMP in the absence of p53.
Our current hypothesis that interference with the cAMP signaling pathway in cancer cells would potentiate the effect of DNA-damaging therapeutic agents is based on our results obtained from BCP-ALL cell lines and primary BCP-ALL cells, as well as on the assumption that malignant B cells, such as BCP-ALLs, have higher cAMP levels than their normal counterparts. Except for one study in which several ALL cell lines, including Reh, were shown to express higher cAMP levels than normal resting B lymphocytes,50 there are no comprehensive data available on the cAMP content of either BCP-ALL cell lines or primary cells. Therefore, to verify our hypothesis, a thorough study on the cAMP status in cancer cells in general, and in primary BCP-ALL cells in particular, is required.
Several studies have reported a direct apoptosis-promoting effect of cAMP in lymphocytes. In particular, in circulating B cells and B chronic lymphocytic leukemia cells, cAMP-inducing agents have been shown to increase spontaneous apoptosis or potentiate chlorambucil-induced apoptosis.51-53 On the basis of these findings, cAMP-inducing agents have been proposed as potential novel therapeutics.54 The results presented in our study should, however, call for caution regarding the use of cAMP-elevating agents in this respect. Our data indicate that increasing cAMP levels in cancer cells might be disadvantageous, at least in the context of therapeutic regimens that use DNA-damaging agents.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Howard L. Liber (Colorado State University) for TK6 and WTK1 cell lines and Dr Murielle Masson (ESBS, Illkirch, France) for the pXJ-E6 plasmid.
This work was supported by grants from the Norwegian Cancer Society, the Jahre Foundation, Blix Foundation, Freia Foundation, and Rachel and Otto Kr. Bruum's Foundation.
Authorship
Contribution: E.H.N. designed the research, performed experiments, analyzed data, and wrote the paper; H.W.F. contributed vital reagents and helped with data review and writing of the paper; E.R. contributed patient materials and helped with data review; H.K.B. designed the research, analyzed data, and wrote the paper; and S.N. provided the concept, designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Soheil Naderi, Department of Biochemistry, Institute of Basic Medical Sciences, University of Oslo/Universitetet i Oslo, PO Box 1112, Blindern, N-0317 Oslo, Norway; e-mail: soheil.naderi@basalmed.uio.no.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal