Abstract
Interleukin-17 (IL-17)–secreting CD8+ T cells have been described, but they have not been thoroughly studied and they do not have a known role in cancer immunotherapy. We skewed CD8+ T cells to secrete IL-17 through priming in Th17-polarizing conditions. IL-17–producing CD8+ T cells demonstrated reduced expression of Eomes and diminished cytolytic differentiation in vitro. However, after adoptive transfer, these cells converted to interferon-γ–producing effector cells and mediated regression of large, established tumors. This improved antitumor immunity was associated with increased expression of IL-7R-alpha, decreased expression of killer cell lectin-like receptor G1, and enhanced persistence of the transferred cells. This report is the first description of a cancer therapy with IL-17–secreting CD8+ T cells. These findings have implications for the improvement of CD8+ T cell–based adoptive immunotherapy.
Introduction
Infusion of tumor-reactive T cells is emerging as an effective treatment for Epstein-Barr virus–associated malignancies and for melanoma.1-3 T cells with reactivity against tumor antigens can be generated through T-cell receptor (TCR) gene engineering,4 expanded in vitro, and administered to patients.5 This approach permits not only targeting of any antigen for which a TCR can be isolated but also selection and induction of T-cell subsets with optimal therapeutic potential. Identification of these subsets is a critical challenge in the advance of cancer immunotherapy.6,7
Th17-lineage CD4+ T cells, which express the transcription factor ROR-γt and produce the proinflammatory cytokine interleukin-17 (IL-17), demonstrate enhanced antitumor immunity.8,9 IL-17–producing CD8+ T cells have been identified in mice and in humans,10-13 and they can be generated through in vitro priming with Th17-polarizing cytokines.14 However, a role for these cells in antitumor immunity has not been described.
CD4+ T cells can differentiate into lineages with diverse effector functions. In contrast, CD8+ T cells, through the redundant expression of Eomes and T-bet, are fated to develop into cytolytic effector cells that produce IFN-γ and express granzyme B and perforin.11,15 It is not known if induction of IL-17 production in this cytolytic lineage would enhance its ability to mediate tumor destruction. Interestingly, the improved antitumor immunity observed with Th17 cells appears to be IL-17–independent, suggesting that it is not IL-17 secretion per se, but rather another characteristic conferred by Th17-polarization, that improves their function.8
To study tumor therapy with type 17–skewed CD8+ T cells, we used the pmel-1 model of adoptive immunotherapy of B16 melanoma.16 This model reproduces the clinical challenge of targeting the shared tumor/self-antigen, gp100, in poorly immunogenic, vascularized B16 melanoma tumors. Pmel-1, gp100-specific, TCR-transgenic CD8+ T cells that were primed and expanded before infusion were used to parallel human peripheral blood lymphocytes that are TCR transduced and expanded for clinical therapy.5
Methods
Mice and tumor lines
Pmel-1/Thy1.1 TCR-transgenic mice have been described previously.16,17 They were housed in the National Institutes of Health (NIH) Clinical Research Center vivarium and maintained in compliance with the NIH Animal Care and Use Committee. C57BL/6 mice were purchased from The Jackson Laboratory. The B16F10 and MCA205 tumor lines were obtained from the National Cancer Institute (NCI) tumor repository. All animal studies were approved by the NCI Animal Care and Use Committee.
In vitro assays
Type 17 skewing was accomplished by priming pmel-1/Thy1.1 splenocytes with 1 μM hgp10025-33 in rmIL-6 (5 ng/mL) and recombinant human transforming growth factor-β (rhTGF-β; 10 ng/mL; R&D Systems), and anti–IFN-γ (10 μg/mL; eBioscience). Beginning 2 days after priming, cell cultures were expanded with rhIL-2 (30 IU/mL; Novartis). Control cells were primed and expanded with IL-2 alone. Assays were conducted 5 to 7 days after priming. Cytokine, 51Cr release, real-time polymerase chain reaction, and cytofluorometric analyses were performed as described.17-19
Adoptive cell transfer
Tumor therapy was performed as described.18 The numbers of transferred cells present in spleens of treated mice were calculated by multiplying the total number of viable splenocytes by the frequency of Thy1.1+ CD8+ cells.
Results and discussion
Skewing of tumor-specific CD8+ T cells to produce IL-17
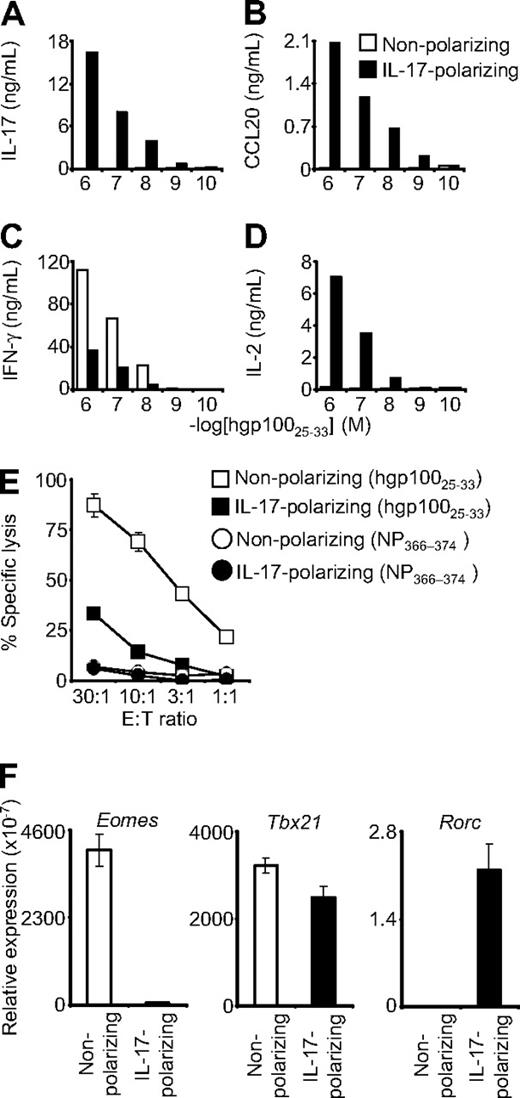
Pmel-1 CD8+ T cells were antigen-primed in a cocktail of IL-6, TGF-β, and anti–IFN-γ (“IL-17 conditions”) that can promote the differentiation of CD4+ T cells into the Th17 lineage.20 The cells were expanded with IL-2 and then tested for cytokine secretion in overnight coculture assays. Cells primed in IL-17 conditions demonstrated antigen-specific release of IL-17, but those primed in nonpolarizing conditions showed minimal or no IL-17 secretion (Figure 1A). Similarly, CCL20 (MIP-3-α), a chemotactic factor that is highly up-regulated in Th17 but not nonpolarized or Th1 cells,8,21 was released by CD8+ T cells primed in IL-17 conditions but not in the nonpolarizing conditions (Figure 1B). These data suggested that the pmel-1 CD8+ T cells could indeed be skewed to a Th17-like, IL-17–producing state.
Type 17–polarized CD8+ T cells produced IL-17 and were diverted from cytolytic differentiation. Pmel-1/Thy1.1 CD8+ T cells were primed in IL-17–polarizing conditions or in nonpolarizing conditions and then expanded with IL-2. (A-D) The cells were then cocultured overnight with target splenocytes pulsed with hgp10025-33 peptide. Production of IL-17, CCL20, IFN-γ, and IL-2 was determined by enzyme-linked immunosorbent assay. (E) Four-hour 51Cr release cytolysis assays were performed. Target cells were pulsed with the peptide indicated in the figure legend. Error bars indicate the SEM. (F) Real-time reverse-transcribed polymerase chain reaction was performed to assess expression of the indicated transcription factors. Expression relative to β-actin is displayed. Error bars represent the SEM.
Type 17–polarized CD8+ T cells produced IL-17 and were diverted from cytolytic differentiation. Pmel-1/Thy1.1 CD8+ T cells were primed in IL-17–polarizing conditions or in nonpolarizing conditions and then expanded with IL-2. (A-D) The cells were then cocultured overnight with target splenocytes pulsed with hgp10025-33 peptide. Production of IL-17, CCL20, IFN-γ, and IL-2 was determined by enzyme-linked immunosorbent assay. (E) Four-hour 51Cr release cytolysis assays were performed. Target cells were pulsed with the peptide indicated in the figure legend. Error bars indicate the SEM. (F) Real-time reverse-transcribed polymerase chain reaction was performed to assess expression of the indicated transcription factors. Expression relative to β-actin is displayed. Error bars represent the SEM.
Diversion of IL-17 skewed cells from canonical cytolytic differentiation
Production of IFN-γ is a quintessential trait of Th1 cells and cytolytic CD8+ T cells. The ability of CD8+ T cells to produce IFN-γ increases with antigen-driven differentiation into fully cytolytic effector cells.22 Conversely, the capacity of CD8+ T cells to release IL-2 is greatest early in postthymic development and decreases with progressive cytolytic differentiation.22 IL-17–skewed CD8+ T cells demonstrated diminished production of IFN-γ but enhanced expression of IL-2 (Figure 1C-D). In addition, they displayed diminished specific killing in chromium release assays (Figure 1E). These findings indicated reduced cytolytic effector function and suggested that, as with diversion of Th17 cells from Th1 development, type 17 CD8+ T cells were skewed away from IFN-γ production and cytolytic differentiation. We tested for a possible molecular basis for this diversion by examining the transcription factors that control T-cell lineage development. Expression of Eomes, the transcriptional master regulator that confers cytolytic differentiation to NK and CD8+ T-cell lineages, was more than 75-fold less in IL-17–skewed cells.15,23 Expression of Tbx21, which encodes T-bet, the primary determinant of Th1 development, was also slightly, but reproducibly, decreased; and consistent with type 17 differentiation, Rorc expression was increased.24 These observations were consistent with the finding that CD8+ T cells skew to an IL-17–producing lineage in Eomes/T-bet double-knockout mice11 ; and taken together, they support the notion that IL-17–skewed cells were diverted from classic cytolytic differentiation. Furthermore, in contrast to the CD4+ T-cell differentiation paradigm, this reduced cytolytic development was associated with markedly decreased expression of Eomes rather than T-bet.
The degree of CD8+ T-cell cytolytic differentiation is particularly important because of the inverse relationship between acquisition of effector function in vitro and the ability to mediate tumor regression in vivo.22 This inverse correlation has been observed with priming in the presence of IL-21, a Th17 cytokine that inhibits Eomes expression and antigen- and IL-2–induced CD8+ T-cell cytolytic differentiation in vitro, resulting in enhanced efficacy of transferred cells in vivo.18 TGF-β and anti–IFN-γ might be playing a role similar to IL-21 in blocking effector development of type 17–skewed CD8+ T cells, resulting in less cytolytic differentiation but more effective cells.
Priming in IL-17 conditions enhanced antitumor immunity
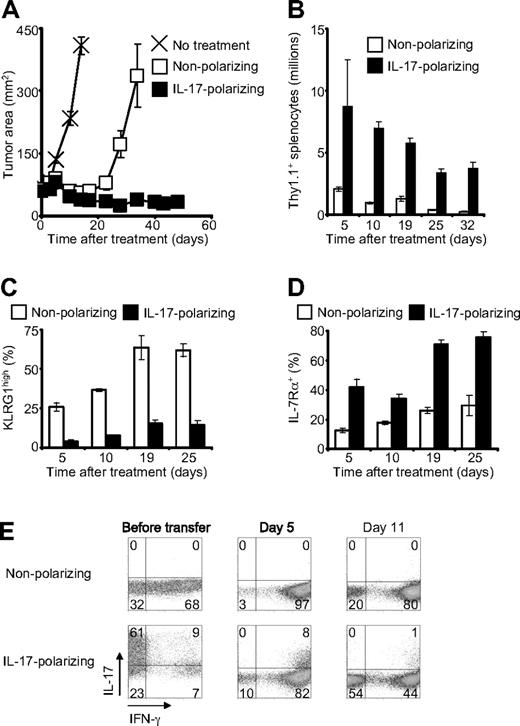
To assess the ability of type 17–skewed cells to cause tumor destruction in vivo, we adoptively transferred pmel-1 CD8+ T cells into mice bearing established, vascularized tumors. The transferred cells were administered in conjunction with specific vaccination and IL-2, which were required for appreciable tumor regression with either nonpolarized or type 17–polarized cells. Cells treated in nonpolarizing conditions induced temporary tumor regression that was followed by recrudescence beginning approximately 20 days after transfer (Figure 2A). In contrast, cells treated in type 17–polarizing conditions mediated sustained antitumor activity throughout the 48-day experiment (Figure 2A). This enhanced antitumor response was associated with increased in vivo expansion and persistence of the transferred cells with more than 16-fold greater cells present 32 days after infusion (Figure 2B). Furthermore, the type 17–skewed cells displayed reduced expression of killer cell lectin-like receptor G1 (Figure 2C), a marker of CD8+ T-cell terminal differentiation, and increased expression IL-7Rα (Figure 2D), a prosurvival cytokine receptor, suggesting a possible mechanism for the increased numbers of these cells. Finally, we examined cytokine production by the transferred cells 5 and 11 days after infusion. IL-17–skewed cells converted to IFN-γ–producing effector cells, but a diminishing minority subset continued to elaborate IL-17 as well as IFN-γ (Figure 2E). This finding suggests that, as with Th17 cells, IFN-γ rather than IL-17 might be the critical cytokine for tumor destruction.8
Type 17–polarized CD8+ T cells mediated enhanced antitumor immunity and demonstrated greater persistence. A total of 106 pmel-1/Thy1.1 CD8+ T cells were adoptively transferred into mice bearing established, vascularized B16F10 melanomas. Recipient mice were pretreated with 5 Gy total body irradiation, and they received adjuvant vaccine and IL-2 in conjunction with cell therapy.16 (A) Serial tumor measurements were obtained and tumor areas were calculated. Error bars indicate the SEM (N = 5). The experiment shown is representative of 3 independent experiments. (B) Expansion and persistence of adoptively transferred cells. The spleens of 3 mice per condition were examined at each time point. Error bars represent the SEM. (C-D) Expression of killer cell lectin-like receptor G1 and IL-7Rα by transferred cells after infusion as determined by flow cytometry gated on Thy1.1+ cells. The data represent 3 spleens per condition at each time point. Error bars represent the SEM. (E) Flow cytometric determination of intracellular cytokines after infusion. The dot plots are gated on Thy1.1+ cells from a pool of 3 spleens per condition at each time point.
Type 17–polarized CD8+ T cells mediated enhanced antitumor immunity and demonstrated greater persistence. A total of 106 pmel-1/Thy1.1 CD8+ T cells were adoptively transferred into mice bearing established, vascularized B16F10 melanomas. Recipient mice were pretreated with 5 Gy total body irradiation, and they received adjuvant vaccine and IL-2 in conjunction with cell therapy.16 (A) Serial tumor measurements were obtained and tumor areas were calculated. Error bars indicate the SEM (N = 5). The experiment shown is representative of 3 independent experiments. (B) Expansion and persistence of adoptively transferred cells. The spleens of 3 mice per condition were examined at each time point. Error bars represent the SEM. (C-D) Expression of killer cell lectin-like receptor G1 and IL-7Rα by transferred cells after infusion as determined by flow cytometry gated on Thy1.1+ cells. The data represent 3 spleens per condition at each time point. Error bars represent the SEM. (E) Flow cytometric determination of intracellular cytokines after infusion. The dot plots are gated on Thy1.1+ cells from a pool of 3 spleens per condition at each time point.
These findings have important implications as we search for the most effective T cells for adoptive immunotherapy. With the ability to confer tumor specificity through genetic engineering, we also gain the capacity to select optimal T-cell subsets and to manipulate the growth, development, and gene expression programs of these cells through priming signals and the cytokine milieu.5-7 This study suggests that type 17 skewing of CD8+ T cells for adoptive immunotherapy improves their efficacy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rosanne Spolski of the National Heart Lung and Blood Institute Laboratory of Molecular Immunology for her assistance.
This work was supported by the NCI, Bethesda, MD (through the intramural program).
A.K.'s current affiliation is Department of Immunology, Netherlands Cancer Institute, Amsterdam, The Netherlands; C.M.P.'s current affiliation is Abramson Family Cancer Research Institute, University of Pennsylvania, Philadelphia; and L.S.-P.'s current affiliation is Robert Preston Tisch Brain Tumor Center, Duke University, Durham, NC.
National Institutes of Health
Authorship
Contribution: C.S.H. designed and performed the experiments, analyzed the data, created the figures, and wrote the manuscript; A.K. designed and performed the experiments and wrote the manuscript; C.M.P., L.C., L.S.-P., B.H., C.W., and P.M. designed and performed the experiments; Z.A.B. performed experiments and edited the manuscript; and N.P.R. directed the project, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pawel Muranski, NCI, NIH, Clinical Research Center, Rm 3-5816, 10 Center Dr, Bethesda, MD 20892; e-mail: Pawel_Muranski@nih.gov.
References
Author notes
*C.S.H., A.K., and C.M.P. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal