Abstract
In acute promyelocytic leukemia (APL), differentiation therapy with all-trans retinoic acid (ATRA) and/or arsenic trioxide can induce a differentiation syndrome (DS) with massive pulmonary infiltration of differentiating leukemic cells. Because chemokines are implicated in migration and extravasation of leukemic cells, chemokines might play a role in DS. ATRA stimulation of the APL cell line NB4 induced expression of multiple CC-chemokines (CCLs) and their receptors (> 19-fold), resulting in increased chemokine levels and chemotaxis. Induction of CCL2 and CCL24 was directly mediated by ligand-activated retinoic acid receptors. In primary leukemia cells derived from APL patients at diagnosis, ATRA induced chemokine production as well. Furthermore, in plasma of an APL patient with DS, we observed chemokine induction, suggesting that chemokines might be important in DS. Dexamethasone, which efficiently reduces pulmonary chemokine production, did not inhibit chemokine induction in APL cells. Finally, chemokine production was also induced by arsenic trioxide as single agent or in combination with ATRA. We propose that differentiation therapy may induce chemokine production in the lung and in APL cells, which both trigger migration of leukemic cells. Because dexamethasone does not efficiently reduce leukemic chemokine production, pulmonary infiltration of leukemic cells may induce an uncontrollable hyperinflammatory reaction in the lung.
Introduction
Acute promyelocytic leukemia (APL) is characterized by a specific t(15;17) chromosomal translocation, which fuses the promyelocytic leukemia (PML) gene on chromosome 15 to the retinoic acid receptor-α (RARA) gene on chromosome 17. This translocation results in blockage of terminal granulocytic differentiation at the promyelocytic stage.1,2 Treatment with high dosage of the RARA ligand all-trans retinoic acid (ATRA) relieves this blockage, resulting in terminal differentiation of the APL blasts. Presently, standard therapy for patients with newly diagnosed APL consists of a combination of ATRA and anthracycline-based chemotherapy, which results in the induction of a complete remission in more than 90% of the patients and a 5-year overall survival rate of more than 80%.3-11 In addition, arsenic trioxide (ATO) treatment has been shown to be highly effective in relapsed APL patients and has also been used successfully as a single agent or in combination with ATRA in newly diagnosed APL patients.12-21
Despite the high cure rates, induction mortality is a still a problem in APL. In a large series of 732 APL patients who received ATRA plus idarubicin, induction mortality was 9%. The most common causes of death were hemorrhage, infection, and the differentiation syndrome (DS), formerly known as retinoic acid syndrome.22 DS is reported in 2.5% to 31% of the APL patients who receive induction therapy with ATRA and/or ATO.5-7,10,14,16,19,20,23-30 DS is not observed during consolidation or maintenance therapy with ATRA and/or ATO in APL patients, nor is DS observed during ATRA treatment in non-APL malignancies, implicating that the APL cells play a crucial role in the development of DS.6,7,14,20,23,26,27,31 DS is characterized by respiratory distress, unexplained fever, weight gain, interstitial pulmonary infiltrates, pleural or pericardial effusions, hypotension, and acute renal failure. The diagnosis DS is made on clinical grounds by the presence of at least 3 of the aforementioned signs and/or symptoms, in the absence of alternative explanations.10,23,26,27,29 In addition, DS is often associated with the development of hyperleukocytosis, pulmonary edema, generalized edema, headache, and bone pain.10,23,26,27,29,30 The diagnosis DS has proven to be difficult because none of the aforementioned symptoms is pathognomonic of the syndrome and can be the result of concurrent medical problems, such as bacteremia, sepsis, pneumonia, pulmonary hemorrhage, or congestive heart failure.32,33 Therefore, DS may be underdiagnosed as well as overdiagnosed.
In DS an excessive systemic inflammatory response is observed which can result in massive tissue infiltration of differentiating APL cells. The lung is one of the most relevant target organs of the inflammatory response in DS, illustrated by marked histopathologic changes and respiratory problems, requiring mechanical ventilation in 15% to 55% of the APL patients with DS.10,23,26,27,29,34,35 Furthermore, the excessive inflammatory response can induce a life-threatening capillary-leak syndrome and multiorgan failure.10,23,26,27,36 Although early recognition of DS and prompt treatment with high-dose dexamethasone have reduced the DS-related mortality significantly, still 15% of the induction deaths in APL are associated with DS.5-7,10,22,24-28 Furthermore, the development of DS has additional implications, such as an increased morbidity (eg, increased risk of hemorrhage, higher incidence of thrombosis, increased rate of grade 3 or 4 hepatotoxicity), and may be associated with a higher risk of relapse.10,22,28,29
The pathogenic mechanism of the hyperinflammatory cascade in DS is not fully understood. Two different mechanisms play an important role in the development of DS: the migration of APL cells to the lung and the differentiation of APL cells. The initial migration of APL cells to the lung seems to be triggered by alveolar chemokine secretion.34,37,38 ATRA is able to significantly increase the expression and production of specific chemokines (CCL2 and CXCL8) by alveolar epithelial cells in vitro and in vivo, resulting in increased migration of APL cells toward alveolar epithelial cells.34,37,38 Suppression of alveolar chemokine secretion with dexamethasone or neutralization with specific chemokine antibodies resulted in reduction of the migration.34,37,38 Differentiation of APL cells may be an additional crucial factor in the development of DS because it seems to be required for the pulmonary infiltration of APL cells, observed in DS.34,37-39 In this respect, Tsai et al showed that ATRA stimulation of APL cells significantly increased the migration of APL cells toward alveolar epithelial cells in vitro.37,38 In addition, in a murine APL model, transplantation of ATRA-treated APL cells resulted in the development of DS with pulmonary infiltration and lung edema, which was not observed when untreated APL cells or ATRA-resistant APL cells were used for transplantation.34
Differentiation induction of APL cells is associated with increased expression of specific adhesion molecules and inflammatory cytokines, which may promote activation, migration, and adhesion of these cells. Furthermore, up-regulation of specific chemokines (CCL2 and CXCL8) has been reported during differentiation induction of APL cells.33,34,37-48 Because chemokines coordinate the development, differentiation, and trafficking of leukocytes during inflammatory reactions and mediate the migration of leukemic cells, increased production of chemokines by differentiating APL cells may be important for the development of DS.49 Therefore, we examined the role of chemokines in the development of DS.
Methods
Cell culture and in vitro differentiation induction
NB4 cells, a retinoic acid–sensitive human APL cell line with the typical t(15;17) chromosomal translocation, were cultured in RPMI 1640 culture medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (MP Biomedicals).50 Primary leukemia cells were isolated from bone marrow or peripheral blood of 5 APL patients at diagnosis after informed consent. Mononuclear cells were isolated by a Ficoll-1077 (GE Healthcare) gradient and stored in liquid nitrogen. After thawing, leukemic cells were cultured in RPMI 1640 medium, supplemented with 20% FBS and 1% penicillin/streptomycin. Cultures were incubated at 37°C in a 5% CO2 completely humidified atmosphere. Leukemic cells were cultured in the presence or absence of ATRA (Sigma-Aldrich) for 0 to 48 hours. NB4 cells were cultured in the presence or absence of ATRA, ATO (Sigma-Aldrich), dexamethasone (Sigma-Aldrich), or cycloheximide (Sigma-Aldrich) for 0 to 96 hours. ATRA, ATO, and dexamethasone were used at a final concentration of 10−6 M. Cycloheximide was added 30 minutes before ATRA at a final concentration of 50 μg/mL.
Morphology and flow cytometric analysis
NB4 cells, treated with ATRA and/or ATO, were harvested at the indicated time points. To evaluate the morphologic status of NB4 cells, May-Grünwald-Giemsa (MGG) staining of cytospins was performed at the indicated time points. Morphologic analysis was performed using the Axioskop I microscope (Plan-NEOFLUAR 40×/0.75 objective; Zeiss). Images were acquired using the Sony 3CCD color video camera (DXC-950P; Sony). Microsoft Photo Editor Version 3.01 was used for image acquisition (Microsoft Corporation). To analyze the differentiation of NB4 cells during ATRA and/or ATO induction, cells were stained with a phycoerythrin-conjugated human CD11b antibody (Dako North America). To analyze apoptosis of NB4 cells during ATRA and/or ATO induction, cells were stained with a fluorescein isothiocyanate–conjugated annexin V antibody (VPS Diagnostics) in combination with a propidium iodide antibody (P-4170; Sigma-Aldrich). Stained cells were analyzed with CXP software (Beckman Coulter) on a Beckman Coulter Cytomics Flow Cytometer 500 (Beckman Coulter).
APL patients, induction therapy, and diagnosis of DS
After informed consent was obtained in accordance with the Declaration of Helsinki, we obtained plasma from newly diagnosed APL patients before and during treatment according to the AIDA-2000/P protocol.5,51 Institutional review board approval for these studies was obtained from the Radboud University Nijmegen Medical Center according to local laws and regulations. Induction therapy was composed of ATRA (45 mg/m2 per day, from day 1 until day 30) and idarubicin (12 mg/m2 per day on days 2, 4, 6, and 8).5,51 Prednisone (0.5 mg/kg per day) was added to the treatment from day 1 until the end of ATRA therapy to prevent DS. Diagnosis of APL was based on cell morphology, cytogenetics, immune phenotyping, and polymerase chain reaction (PCR) for PML-RAR-α. Diagnosis of DS was made according to the presence of at least 3 of the following signs or symptoms described by Frankel et al23 : respiratory distress, weight gain, unexplained fever, pulmonary infiltrates, pleuropericardial effusions, hypotension, and acute renal failure. The presence of a high white blood cell (WBC) count, headache, bone pain, pulmonary edema, or generalized edema was considered as supporting evidence for the diagnosis of DS.10,27,29 The diagnosis of disseminated intravascular coagulation was based on the presence of hemorrhage, thrombocytopenia, low fibrinogen concentrations, and high levels of fibrin degradation products (D-dimer). WBC counts and coagulation parameters (including fibrinogen, D-dimer, and platelet counts) of the patients at diagnosis and during the first 2 weeks of induction therapy are described in detail in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Microarray and data analysis
Total RNA was isolated from NB4 cells before and after differentiation induction with ATRA using RNA-Bee (Iso-Tex Diagnostics). A second purification step of the total RNA was performed using sodium acetate (0.5M; Calbiochem-Novabiochem) and ethanol (96%; Merck KGaA). RNA quality was tested using the Agilent 2100 Bioanalyzer (Agilent Technologies). A total of 5 μg of total RNA from each sample was used for hybridization on the GeneChip Human Exon 1.0 sense target Array containing 1.4 million probe sets (Affymetrix) according to the manufacturer's instructions. Gene level expression data were calculated for the CORE transcripts with help of Affymetrix Expression Console software using sketch-quantile normalization, PM-GCBG background correction, and summarization with the iterPLIER (Probe Logarithmic Intensity Error Estimation) algorithm. For all arrays, the AUC score was more than 0.90, indicating a good separation of the positive controls from the negative controls. Pearson correlation was used to check the correspondence pattern between time points. For each time point and for each gene, the fold change with respect to the expression at time point t = 0 hour was calculated. Genes encoding for CC-chemokines (CCLs) with more than a 5-fold change compared with time point t = 0 hour were selected for validation. All microarray data have been deposited in the GEO public database under accession number GSE18397.
Quantitative PCR analysis
RNA was isolated from NB4 cells using RNA-Bee (Iso-Tex Diagnostics). RNA from leukemic cells (derived from 5 APL patients) was isolated using the mini RNA Isolation II kit (Zymo Research). For complementary DNA (cDNA) synthesis 1 μg of total RNA from NB4 cells and 300 to 600 ng of total RNA from leukemic cells was used. To measure CCL-mRNA expression, Premixed Taqman Gene Expression assays were used (CCL1: Hs00171072_m1, CCL2: Hs00234140_m1, CCL3: Hs00234142_m1, CCL4: Hs00237011_m1, CCL7: Hs00171147_m1, CCL20: HS00171125_m1, CCL22: Hs00171080_m1, CCL24: Hs00171082_m1, Applied Biosystems). As a reference gene, a premixed assay for GAPDH was used (4326317E, Applied Biosystems). Quantitative PCRs contained 2.5 μL of cDNA, 1× TaqMan Universal PCR Master Mix (58003365-01; Applied Biosystems), 1× TaqMan Gene Expression Assay (Applied Biosystems). The 25-μL reactions were incubated in a 96-well plate for 10 minutes at 95°C, followed by 45 cycles of 10 seconds at 95°C and 1 minute at 60°C. Quantitative PCRs were performed on the ABI/PRISM 7700 or 7900 Sequence Detection system (ABI/PE).
Protein level measurements
Ethylenediaminetetraacetic acid (EDTA)–plasma of 3 APL patients and cell-culture supernatant of NB4 cells and leukemic cells (derived from 5 APL patients) were stored at −20°C and used for quantification of CCLs. Protein levels of CCL1, CCL20, and CCL22 were quantified using Quantikine human enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). CCL24 was quantified using a Quantikine human ELISA kit (R&D Systems) or a RayBio human ELISA kit (RayBiotech). CCL2, CCL4, and CCL7 were quantified using Biosource antibody bead kits (Biosource International) for the cytometric bead-based immunoassay system (Luminex). All assays were performed according to the manufacturer's protocols. The detection limits were as follows: for CCL20, 0.47 pg/mL; for CCL1, 0.71 pg/mL; for CCL24, 2.0 to 2.5 pg/mL; for CCL2 and CCL4, 10 pg/mL; for CCL7, 15 pg/mL; for CCL22, 62.5 pg/mL.
Migration assay
The chemoattractive effect of ATRA-treated APL cells on WBCs was evaluated using transwell polycarbonate inserts (6.5-mm diameter) with 5-μm pores (Costar Corning). Blood from healthy donors was drawn in EDTA. WBCs were isolated with a 1085 g/L Percoll gradient (Pharmacia Fine Chemical) or erythrocyte lysis buffer (140mM NH4Cl, 9mM KHCO3, 0.9mM Na2-EDTA, pH 7.4; Merck KGaA). WBCs were resuspended in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin to a cell concentration of 2 to 6 × 106/mL. This cell suspension was incubated for 1 hour at 37°C and 5% CO2. A total of 200 μL of the WBC suspension was added to the upper compartment. To the lower compartment, 750 μL of NB4 cell-culture supernatant (before or after ATRA stimulation) was added. WBCs were allowed to migrate for 45 to 55 minutes at 37°C and 5% CO2. Next, the inserts were removed and WBCs that had migrated to the lower compartment were counted under the microscope. Migration experiments with NB4 cell-culture supernatants were performed 4 times. Statistical significance between time points was determined using the Student t test.
Results
ATRA induces CCL expression and production in NB4 cells
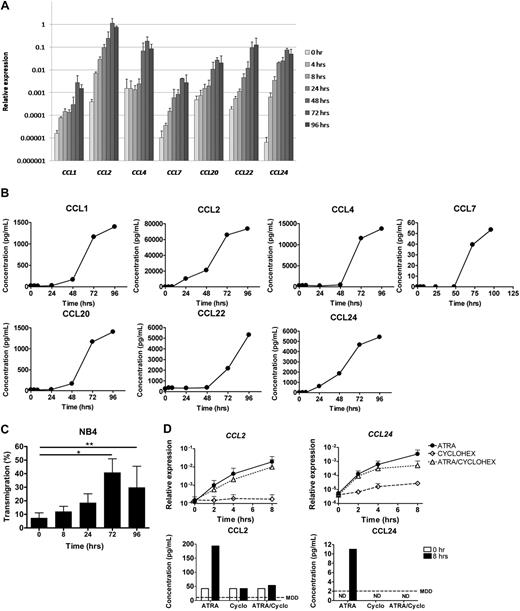
We evaluated the expression profiles of chemokines in the APL cell line NB4 during ATRA-induced differentiation using the GeneChip Human Exon 1.0 sense target Array (Affymetrix). Expression of 8 of 23 CCLs was up-regulated more than 5-fold after ATRA stimulation (Table 1). The expression of several CC-chemokine receptors (CCRs) was up-regulated more than 5-fold as well (Table 1). We focused on the effect of ATRA on CCL expression and confirmed induction of 7 of these CCLs at the mRNA level using quantitative PCR (57- to 11 502-fold; Figure 1A). CCL3 showed an induction of less than 5-fold by quantitative PCR (data not shown). Subsequently, we verified whether the up-regulation at the mRNA level also translated into increased protein levels in supernatant of NB4 cells using ELISA. Protein levels of all 7 CCLs increased significantly after ATRA stimulation (Figure 1B).
ATRA induces expression of CCLs and CCRs in NB4 cells
| . | Gene expression intensity before ATRA, AU . | Maximum gene expression intensity after ATRA, AU . | Fold change . |
|---|---|---|---|
| CCL2 | 71 | 10 285 | 151 |
| CCL22 | 18 | 1822 | 104 |
| CCL24 | 13 | 969 | 78 |
| CCL4 | 14 | 896 | 61 |
| CCL20 | 72 | 1441 | 21 |
| CCL3 | 181 | 3576 | 20 |
| CCL1 | 42 | 754 | 19 |
| CCL7 | 19 | 108 | 6 |
| CCR1 | 48 | 1331 | 28 |
| CCR2 | 41 | 851 | 21 |
| CCR3 | 25 | 489 | 19 |
| . | Gene expression intensity before ATRA, AU . | Maximum gene expression intensity after ATRA, AU . | Fold change . |
|---|---|---|---|
| CCL2 | 71 | 10 285 | 151 |
| CCL22 | 18 | 1822 | 104 |
| CCL24 | 13 | 969 | 78 |
| CCL4 | 14 | 896 | 61 |
| CCL20 | 72 | 1441 | 21 |
| CCL3 | 181 | 3576 | 20 |
| CCL1 | 42 | 754 | 19 |
| CCL7 | 19 | 108 | 6 |
| CCR1 | 48 | 1331 | 28 |
| CCR2 | 41 | 851 | 21 |
| CCR3 | 25 | 489 | 19 |
mRNA expression of CCLs and CCRs was determined in total RNA from NB4 cells cultured with ATRA (10−6 M) for 0, 4, 8, 24, 48, 72, and 96 hours using the GeneChip Human Exon 1.0 ST Array. The table represents chemokines and receptors with at least 5-fold up-regulation of the gene expression intensity in arbitrary units (AUs) after ATRA treatment.
ATRA induces expression of CCLs in NB4 cells. NB4 cells were cultured with ATRA (10−6 M) for 0, 4, 8, 24, 48, 72, and 96 hours. (A) CCL mRNA expression (mean ± SD of 3 independent experiments) was determined at the indicated time points by quantitative PCR. Expression, relative to GAPDH, is plotted. (B) CCL protein levels (mean of 2 independent experiments) were determined in supernatant of NB4 cells at the indicated time points using ELISA. (C) The effect of ATRA on chemoattraction of normal WBCs toward the supernatant of ATRA-stimulated NB4 cells. The percentages of migrated WBCs (mean ± SD of 4 independent experiments) were determined before and 8, 24, 72, and 96 hours after ATRA stimulation of NB4 cells, using a transwell system. *P < .001, **P < .05 compared with supernatant of untreated NB4 cells. (D) Direct regulation of CCL2 and CCL24 by ligand-activated retinoic acid receptors in NB4 cells. NB4 cells were cultured with ATRA alone (10−6 M), with cycloheximide alone (50 μg/mL), or with the combination of ATRA and cycloheximide for 0, 2, 4, and 8 hours. mRNA expression of CCL2 and CCL24 (top panels, mean ± SD of 3 independent experiments) was determined at the indicated time points by quantitative PCR. Expression, relative to GAPDH, is plotted. Protein levels of CCL2 and CCL24 (bottom panels) were measured in supernatant of NB4 cells before and 8 hours after treatment with ATRA and/or cycloheximide using ELISA. ND indicates not detectable; and MDD, minimal detectable dose, according to the manufacturer.
ATRA induces expression of CCLs in NB4 cells. NB4 cells were cultured with ATRA (10−6 M) for 0, 4, 8, 24, 48, 72, and 96 hours. (A) CCL mRNA expression (mean ± SD of 3 independent experiments) was determined at the indicated time points by quantitative PCR. Expression, relative to GAPDH, is plotted. (B) CCL protein levels (mean of 2 independent experiments) were determined in supernatant of NB4 cells at the indicated time points using ELISA. (C) The effect of ATRA on chemoattraction of normal WBCs toward the supernatant of ATRA-stimulated NB4 cells. The percentages of migrated WBCs (mean ± SD of 4 independent experiments) were determined before and 8, 24, 72, and 96 hours after ATRA stimulation of NB4 cells, using a transwell system. *P < .001, **P < .05 compared with supernatant of untreated NB4 cells. (D) Direct regulation of CCL2 and CCL24 by ligand-activated retinoic acid receptors in NB4 cells. NB4 cells were cultured with ATRA alone (10−6 M), with cycloheximide alone (50 μg/mL), or with the combination of ATRA and cycloheximide for 0, 2, 4, and 8 hours. mRNA expression of CCL2 and CCL24 (top panels, mean ± SD of 3 independent experiments) was determined at the indicated time points by quantitative PCR. Expression, relative to GAPDH, is plotted. Protein levels of CCL2 and CCL24 (bottom panels) were measured in supernatant of NB4 cells before and 8 hours after treatment with ATRA and/or cycloheximide using ELISA. ND indicates not detectable; and MDD, minimal detectable dose, according to the manufacturer.
Next, we investigated whether the supernatant of ATRA-treated NB4 cells could induce chemoattraction of leukocytes in a transwell system. In 4 independent experiments, 7% (± 4%) of healthy WBCs migrated from the insert toward the lower compartment with supernatant of untreated NB4 cells within 45 to 55 minutes. When supernatant collected 72 or 96 hours after ATRA stimulation was used, the percentage of transmigrating WBCs increased significantly to 41% (± 10%, P = .001) and 30% (± 16%, P = .03), respectively (Figure 1C). Overall, we observed an increased chemoattractive capacity of supernatant of ATRA-treated NB4 cells compared with untreated NB4 cells, in parallel with the increased chemokine production (Figure 1A-C).
Interestingly, the expression of CCL2 and CCL24 was already more than 5-fold up-regulated within 4 hours after ATRA stimulation (18- and 98-fold, respectively, Figure 1A), suggesting that these chemokines may be directly regulated by ligand-activated retinoic acid receptors. The early up-regulation of these chemokines resulted in increased protein levels in the supernatant 8 hours after ATRA stimulation (6- and 8-fold, respectively, Figure 1B). To investigate whether the induction of CCL2 and CCL24 gene expression was directly regulated by ligand-activated retinoic acid receptors or, alternatively, was regulated by intermediate proteins that were induced in response to ATRA, NB4 cells were treated with the protein translation inhibitor cycloheximide, before the addition of ATRA. Despite the addition of cycloheximide, CCL2 and CCL24 mRNA expression was up-regulated (13- and 76-fold, respectively) 4 hours after ATRA stimulation (Figure 1D). As expected, CCL2 and CCL24 protein levels were not induced, indicating that protein translation was indeed effectively inhibited (Figure 1D). These results indicate that CCL2 and CCL24 are directly regulated by ligand-activated retinoic acid receptors.
Dexamethasone does not abrogate ATRA-induced CCL expression
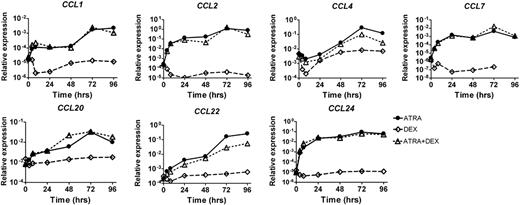
Dexamethasone is used for the prevention and treatment of DS. Therefore, we investigated the effect of dexamethasone on the ATRA-induced chemokine expression in APL in vitro. NB4 cells were treated with ATRA, dexamethasone, or both. Subsequently, chemokine expression was measured by quantitative PCR. We observed induction of CCL expression by ATRA, regardless of the addition of dexamethasone. In the absence of ATRA, the expression of these CCLs was not induced (Figure 2). These results show that dexamethasone does not abrogate the induction of CCLs in ATRA-stimulated APL cells in vitro.
Dexamethasone does not abrogate ATRA-induced CCL expression in NB4 cells. NB4 cells were cultured with ATRA alone (10−6 M), with dexamethasone alone (10−6 M), or with the combination of ATRA and dexamethasone for 0, 4, 8, 24, 48, 72, and 96 hours. CCL mRNA expression was determined at the indicated time points by quantitative PCR. Expression relative to GAPDH is plotted.
Dexamethasone does not abrogate ATRA-induced CCL expression in NB4 cells. NB4 cells were cultured with ATRA alone (10−6 M), with dexamethasone alone (10−6 M), or with the combination of ATRA and dexamethasone for 0, 4, 8, 24, 48, 72, and 96 hours. CCL mRNA expression was determined at the indicated time points by quantitative PCR. Expression relative to GAPDH is plotted.
ATRA induces CCL expression in APL patients in vitro and in vivo
Next, we investigated whether the 7 CCLs that were induced in NB4 cells were also induced in primary leukemia cells from APL patients. Leukemic cells derived from 5 APL patients at diagnosis were induced with ATRA in vitro. CCL2 was the only chemokine that was consistently up-regulated on mRNA and protein level in all 5 patients during ATRA induction. mRNA levels of CCL2 were 3- to 6-fold up-regulated 48 hours after ATRA stimulation (Figure 3A), resulting in a 2- to 14-fold up-regulation of CCL2 protein levels in the supernatant of these cells (Figure 3B).
ATRA induces CCL expression in APL patients in vitro and in vivo. (A) Primary leukemia cells derived from 5 different APL patients at diagnosis were cultured with ATRA (10−6 M) for 0, 8, and 48 hours CCL mRNA expression was determined at the indicated time points by quantitative PCR. Expression relative to GAPDH is plotted. (B) CCL2 protein levels were determined in supernatant of the leukemic cells at the indicated time points using ELISA. (C) Protein levels of CCLs were determined in plasma of 3 APL patients before and during induction therapy with ATRA (45 mg/m2 per day), idarubicin (Ida, 12 mg/m2 per day on days 2, 4, 6, and 8), and prednisone (PRED, 0.5 mg/kg per day) using ELISA. Patient A (left panel) and patient B (middle panel) had no signs of DS. Patient C (right panel) developed a DS within 24 hours after initiation of induction therapy. (D) Clinical course of APL patient C who developed DS. The patient presented with a high WBC count (8.2 × 109/L). One day after onset of induction therapy, DS manifested with mild respiratory distress, pleural effusion, and diffuse pulmonary infiltrates on chest X-ray. ATRA was discontinued and dexamethasone (10 mg/12 hours) was administered. A peak WBC count of 17.1 × 109/L (black line) was reached 3 days after start of therapy. Although ATRA therapy was discontinued, the patient developed generalized edema, weight gain (gray line), and headache. Respiratory distress aggravated on days 4 to 5, resulting in an increasing need for oxygen supply. High resolution computed tomography on day 6 showed evident signs of interstitial and alveolar pulmonary edema. After DS completely resolved, ATRA was resumed on day 8 at a 20% dose and gradually increased to a 100% dose at day 12. Dexamethasone was continued until day 11.
ATRA induces CCL expression in APL patients in vitro and in vivo. (A) Primary leukemia cells derived from 5 different APL patients at diagnosis were cultured with ATRA (10−6 M) for 0, 8, and 48 hours CCL mRNA expression was determined at the indicated time points by quantitative PCR. Expression relative to GAPDH is plotted. (B) CCL2 protein levels were determined in supernatant of the leukemic cells at the indicated time points using ELISA. (C) Protein levels of CCLs were determined in plasma of 3 APL patients before and during induction therapy with ATRA (45 mg/m2 per day), idarubicin (Ida, 12 mg/m2 per day on days 2, 4, 6, and 8), and prednisone (PRED, 0.5 mg/kg per day) using ELISA. Patient A (left panel) and patient B (middle panel) had no signs of DS. Patient C (right panel) developed a DS within 24 hours after initiation of induction therapy. (D) Clinical course of APL patient C who developed DS. The patient presented with a high WBC count (8.2 × 109/L). One day after onset of induction therapy, DS manifested with mild respiratory distress, pleural effusion, and diffuse pulmonary infiltrates on chest X-ray. ATRA was discontinued and dexamethasone (10 mg/12 hours) was administered. A peak WBC count of 17.1 × 109/L (black line) was reached 3 days after start of therapy. Although ATRA therapy was discontinued, the patient developed generalized edema, weight gain (gray line), and headache. Respiratory distress aggravated on days 4 to 5, resulting in an increasing need for oxygen supply. High resolution computed tomography on day 6 showed evident signs of interstitial and alveolar pulmonary edema. After DS completely resolved, ATRA was resumed on day 8 at a 20% dose and gradually increased to a 100% dose at day 12. Dexamethasone was continued until day 11.
To determine whether ATRA could also induce chemokine production in vivo, we measured plasma levels of CCLs in 3 APL patients during induction therapy with ATRA, idarubicin, and prednisone (AIDA-2000/P protocol).5,51 All 3 patients presented with hemorrhage, thrombocytopenia (12-31 × 109/L), low to normal fibrinogen concentrations (1410-1640 mg/L), and high levels of fibrin degradation products (D-dimer: 14 720-31 820 ng/mL), which are indicative for disseminated intravascular coagulation, and therefore received multiple transfusions with fresh frozen plasma, platelets, and red blood cells (supplemental Figure 1). In patient A and B, induction therapy was not complicated by DS or hyperleukocytosis (maximum WBC count: 2.1 × 109/L and 1.9 × 109/L, respectively, supplemental Figure 1). In both patients, no evident induction of chemokines was observed in plasma during induction therapy with ATRA and idarubicin (Figure 3C; supplemental Table 1). Patient C presented with a high WBC count at diagnosis (8.2 × 109/L) and developed DS within 24 hours after start of induction therapy with ATRA and prednisone (Figure 3D). The diagnosis of DS was based on development of respiratory distress in combination with diffuse pulmonary infiltrates and pleural effusion on chest X-ray, without any signs of infection. Therefore, ATRA was discontinued and dexamethasone treatment was started immediately. The diagnosis of DS was supported by the development of hyperleukocytosis, weight gain, headache, generalized edema, and pulmonary edema (interstitial and alveolar) without focal infiltrations on high resolution computed tomography scan (Figure 3D). Interestingly, we observed evident induction of multiple chemokines (2- to 5-fold), including CCL2, during induction therapy in the plasma of patient C, which may be associated with the development of the DS (Figure 3C; supplemental Table 1).
ATO induces expression and production of CCL2 and CCL7 in NB4 cells
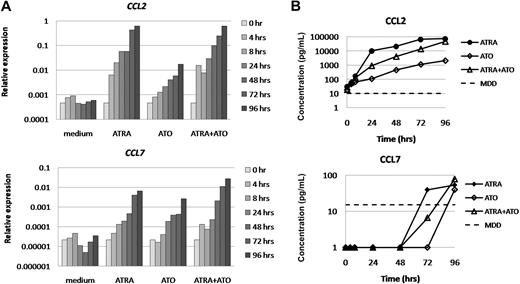
Because DS is also observed during induction therapy with ATO as a single agent or in combination with ATRA, we subsequently investigated the effect of both treatment modalities on chemokine expression in APL.14,16,19,20 NB4 cells were cultured in medium in the presence or absence of ATRA, ATO, or their combination for 96 hours. Morphologic analysis showed that ATRA induced differentiation of NB4 cells, which was confirmed by a significant increase in CD11b expression, measured by flow cytometry (Figure 4A-B). ATO treatment resulted in morphologically apoptotic cells with partial differentiation, as described by Chen et al (Figure 4A).52,53 In addition, flow cytometric analysis showed a significant increase in annexin V–positive and double-positive (annexin V and propidium iodide) cells during ATO treatment, which is a hallmark for apoptosis induction, and a slight increase of CD11b expression (Figure 4B-C). When ATRA and ATO were combined, we observed evident induction of differentiation and apoptosis, as expected (Figure 4).54 Subsequently, we investigated the effect of ATO on the expression of the 7 CCLs that were induced by ATRA in NB4 cells. CCL2 and CCL7 were consistently up-regulated at the mRNA and protein level in ATO-treated NB4 cells. At 96 hours after ATO stimulation, mRNA levels of CCL2 and CCL7 were up-regulated 37- and 124-fold, respectively, resulting in a significant increase of protein levels of both CCLs in supernatant of ATO-treated NB4 cells (Figure 5). The expression of CCL1, CCL4, CCL20, CCL22, and CCL24 was less than 5-fold up-regulated in ATO-treated NB4 cells (supplemental Figure 2). When ATO was combined with ATRA, the expression of all 7 CCLs was induced (32- to 1664-fold, Figure 5; supplemental Figure 2).
Induction of differentiation and apoptosis by ATRA and ATO in NB4 cells. NB4 cells were cultured in medium in the presence or absence of ATRA (10−6 M), ATO (10−6 M), or their combination, for 0, 4, 8, 24, 48, 72, and 96 hours. (A) May-Grünwald-Giemsa staining was performed on cytospins after the indicated treatments for 96 hours. ATRA-treated cells showed typical differentiation features, such as wider cytoplasm and more lobulated nuclei. ATO-treated cells showed evident signs of apoptosis, including fragmentation of the nuclei and formation of apoptotic bodies. Original magnification ×400. (B) After 72 hours of ATRA and/or ATO treatment, CD11b expression was analyzed by flow cytometry to determine differentiation. Mean fluorescence intensity (MFI) of the life-gated cells is plotted. (C) Annexin V and propidium iodide (PI) staining was performed to determine apoptosis after 72 hours of ATRA and/or ATO treatment. Analysis was performed on the ungated cells. The percentages of negative, single-positive, and double-positive cells are depicted.
Induction of differentiation and apoptosis by ATRA and ATO in NB4 cells. NB4 cells were cultured in medium in the presence or absence of ATRA (10−6 M), ATO (10−6 M), or their combination, for 0, 4, 8, 24, 48, 72, and 96 hours. (A) May-Grünwald-Giemsa staining was performed on cytospins after the indicated treatments for 96 hours. ATRA-treated cells showed typical differentiation features, such as wider cytoplasm and more lobulated nuclei. ATO-treated cells showed evident signs of apoptosis, including fragmentation of the nuclei and formation of apoptotic bodies. Original magnification ×400. (B) After 72 hours of ATRA and/or ATO treatment, CD11b expression was analyzed by flow cytometry to determine differentiation. Mean fluorescence intensity (MFI) of the life-gated cells is plotted. (C) Annexin V and propidium iodide (PI) staining was performed to determine apoptosis after 72 hours of ATRA and/or ATO treatment. Analysis was performed on the ungated cells. The percentages of negative, single-positive, and double-positive cells are depicted.
ATO induces expression of CCL2 and CCL7 in NB4 cells. NB4 cells were cultured in medium in the presence or absence of ATRA (10−6 M), ATO (10−6 M), or their combination for 0, 4, 8, 24, 48, 72, and 96 hours. (A) CCL mRNA expression was determined at the indicated time points by quantitative PCR. Expression relative to GAPDH is plotted. (B) CCL protein levels were determined in supernatant of NB4 cells at the indicated time points using ELISA. MDD indicates minimal detectable dose, according to the manufacturer.
ATO induces expression of CCL2 and CCL7 in NB4 cells. NB4 cells were cultured in medium in the presence or absence of ATRA (10−6 M), ATO (10−6 M), or their combination for 0, 4, 8, 24, 48, 72, and 96 hours. (A) CCL mRNA expression was determined at the indicated time points by quantitative PCR. Expression relative to GAPDH is plotted. (B) CCL protein levels were determined in supernatant of NB4 cells at the indicated time points using ELISA. MDD indicates minimal detectable dose, according to the manufacturer.
Discussion
In this study, we show that differentiation induction with ATRA and/or ATO causes a strong up-regulation of chemokine production by APL cells. This is in line with results of Shibakura et al who reported induction of chemokines in APL patients with DS.46 We propose that massive production of chemokines by differentiating APL cells may play an important role in the development of DS. Previously, it was shown that the production of chemokines by alveolar epithelial cells in the lung, which can be enhanced by ATRA, might be implicated in DS.34,37,38 In addition, we observed that differentiation induction causes up-regulation of the chemokine receptors CCR1, CCR2, and CCR3, which can bind to CCL2, CCL7, CCL24, and several other CCLs (Table 1; supplemental Table 2). The induction of chemokine receptors on differentiating APL cells, together with enhanced secretion of chemokines by alveolar epithelial cells, might trigger the migration of leukemic cells toward the lung. Tissue infiltration of differentiating leukemic cells, which massively produce chemokines, might further enhance the migration of leukemic cells from the blood to the tissues and thereby aggravate the inflammatory response in the tissues. This is supported by results from Tsai et al who showed that blockage of specific chemokine receptors on differentiating APL cells reduced the migration of APL cells toward alveolar epithelial cells in vitro.37,38
Corticosteroid therapy may effectively prevent the development of DS when administered at early stages, but the exact mechanism of action is not completely clear.34 In a previous study, we showed that dexamethasone did not affect the induction of terminal differentiation in APL cells.55 In the present study, we demonstrate that dexamethasone does not abrogate the induction of chemokines in the differentiating APL cells either. In line with these results, Tsai et al showed that dexamethasone did not reduce the secretion of CCL2 and CXCL8 by differentiating APL cells in vitro.37,38 On the other hand, the production of chemokines by the alveolar epithelial cells can effectively be diminished by dexamethasone in vitro as well as in vivo, resulting in reduced migration of APL cells toward alveolar epithelial cells.34,37,38 Together, these results indicate that corticosteroids might prevent pulmonary infiltration of APL cells via reduction of the alveolar chemokine secretion and thereby might prevent the development of DS. In a full-blown DS, differentiating APL cells have already invaded target tissues, such as the lung, and the massive chemokine production by these leukemic cells may induce ongoing chemoattraction and inflammation in these tissues. Because dexamethasone does not reduce the chemokine production by differentiating APL cells, corticosteroids may be less effective in the treatment of a full-blown DS. In addition, we show that the expression of 2 strongly induced chemokines (CCL2 and CCL24) is directly regulated by ligand-activated retinoic acid receptors in APL cells, suggesting a direct role for the PML-RAR-α fusion protein in the induction chemokines. The involvement of the PML-RAR-α fusion protein might explain why DS seems to be confined to APL and is not observed during ATRA treatment in non-APL malignancies. Furthermore, this might explain why dexamethasone can reduce the production of chemokines by alveolar epithelial cells, whereas the chemokine induction in APL cells is not affected by dexamethasone.34,37,38
In conclusion, differentiation therapy may cause massive chemokine production in APL cells, in vitro as well as in vivo. We propose a model illustrating the role of chemokine induction in the development of DS (Figure 6). The first component is the migration of differentiating APL cells toward the lung in response to pulmonary chemokine production. The second component is the enhanced migration of leukemic cells in response to chemokines produced by differentiating APL cells infiltrating the lung (Figure 6A). This process can aggravate the hyperinflammatory reaction in the lung. Because the massive chemokine induction in differentiating APL cells is not abrogated by corticosteroids, disruption of this process may be difficult. Therefore, corticosteroid treatment may not be sufficient to reverse a fully developed DS. According to this model, intervention with corticosteroid may be effective in the initiation phase of DS through reduction of the pulmonary chemokine secretion, eliminating the initial trigger for migration of APL cells to the lung (Figure 6B). Thereby, this model underlines the importance of early recognition of DS and prompt treatment with corticosteroids. For a full-blown DS, the optimal treatment approach is not clear. Interventions that directly target the secreted chemokines or their receptors might inhibit the hyperinflammation and chemotaxis driven by the differentiating APL cells. To this end, the application of chemokine (receptor) antagonists might be effective in a full-blown DS.
Model of the role of chemokines and dexamethasone in the DS. Schematic representation of the role of chemokines (A) and dexamethasone (B) in the DS. (A) ATRA forces APL blasts to differentiate into mature granulocytes (MGG staining ×400). (1) In addition, ATRA induces chemokine production by the differentiating APL cells (2) as well as by alveolar epithelial cells (3). Alveolar chemokine secretion triggers the migration of differentiating APL cells to the lung (gray arrows). Enhanced migration of leukemic cells in response to massive chemokine production by the differentiating APL cells infiltrating the lung (black arrows) will aggravate the hyperinflammatory reaction in the lung. (B) Reduction of the alveolar chemokine secretion by dexamethasone (DEX) can eliminate the initial trigger for migration of APL cells to the lung and thereby may prevent pulmonary infiltration of APL cells (gray arrows). Dexamethasone does not abrogate the chemokine induction in APL cells. As soon as differentiating APL cells have infiltrated the lung, the ongoing chemokine production by these cells will enhance migration of leukemic cells toward the lung (black arrows), resulting in a severe hyperinflammatory reaction.
Model of the role of chemokines and dexamethasone in the DS. Schematic representation of the role of chemokines (A) and dexamethasone (B) in the DS. (A) ATRA forces APL blasts to differentiate into mature granulocytes (MGG staining ×400). (1) In addition, ATRA induces chemokine production by the differentiating APL cells (2) as well as by alveolar epithelial cells (3). Alveolar chemokine secretion triggers the migration of differentiating APL cells to the lung (gray arrows). Enhanced migration of leukemic cells in response to massive chemokine production by the differentiating APL cells infiltrating the lung (black arrows) will aggravate the hyperinflammatory reaction in the lung. (B) Reduction of the alveolar chemokine secretion by dexamethasone (DEX) can eliminate the initial trigger for migration of APL cells to the lung and thereby may prevent pulmonary infiltration of APL cells (gray arrows). Dexamethasone does not abrogate the chemokine induction in APL cells. As soon as differentiating APL cells have infiltrated the lung, the ongoing chemokine production by these cells will enhance migration of leukemic cells toward the lung (black arrows), resulting in a severe hyperinflammatory reaction.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Richard Huijbens, Rheumatology Research and Advanced Therapeutics Department, Radboud University Nijmegen Medical Center, for his help with protein level measurements; Robbert van der Voort for critically reading the manuscript; and Christian Gilissen, Department of Human Genetics, Radboud University Nijmegen Medical Center, for his help with analyzing the microarray data.
This work was supported by the Dutch Cancer Society (grant 03-2897).
Authorship
Contribution: M.L. designed and performed research, analyzed data, and wrote the paper; J.L.A.P. designed and performed research and analyzed data; W.M.W., P.C.M.L., and R.P. performed research and analyzed data; P.M. provided patient materials and clinical data; T.J.M.d.W. and B.A.v.d.R. designed research; and J.H.J. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joop H. Jansen, Central Hematology Laboratory, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Geert Grooteplein Zuid 8, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: j.jansen@chl.umcn.nl.
References
Author notes
*M.L. and J.L.A.P. contributed equally to this study.