Abstract
We previously showed that incorporating target sequences for the hematopoietic-specific microRNA miR-142 into an antigen-encoding transgene prevents antigen expression in antigen-presenting cells (APCs). To determine whether this approach induces immunologic tolerance, we treated mice with a miR-142–regulated lentiviral vector encoding green fluorescent protein (GFP), and subsequently vaccinated the mice against GFP. In contrast to control mice, no anti-GFP response was observed, indicating that robust tolerance to the transgene-encoded antigen was achieved. Furthermore, injection of the miR-142–regulated vector induced a population of GFP-specific regulatory T cells. Interestingly, an anti-GFP response was observed when microRNA miR-122a was inserted into the vector and antigen expression was detargeted from hepatocytes as well as APCs. This demonstrates that, in the context of lentiviral vector-mediated gene transfer, detargeting antigen expression from professional APCs, coupled with expression in hepatocytes, can induce antigen-specific immunologic tolerance.
Introduction
Unwanted immune responses are a major medical problem in autoimmunity and gene therapy. Existing approaches to subdue an immune response use nonspecific methods of immune suppression, including corticosteroids, and molecules that block T-cell signaling, proliferation, and function. Developing a means for inducing antigen-specific tolerance would provide a way to overcome the limitations of existing immune modulating agents. Because multiple antigens are likely to be involved in any single autoimmune disorder or cell/tissue transplant, there has been skepticism about antigen-specific therapy.1 However, the emerging knowledge of regulatory T cells (Tregs) has caused a re-examination of this view.2,3 Tregs play a natural role in preventing the development of autoimmunity by homing to the tissue where their cognate antigen is expressed, and suppressing local immune responses through direct inhibition of effector T cells.4,5 The ability of antigen-specific Tregs to control a complex immune response was demonstrated by experiments showing that adoptive transfer of BDC2.5 Tregs, which are specific for a single islet antigen, into nonobese diabetic mice was able to reduce the incidence of diabetes.6
Delivery of simple antigenic molecules, either by protein or gene vector-based administration, offers an attractive platform for tolerance induction in comparison with global immunosuppression. The most clinically advanced antigen-specific therapies involve administration of the soluble form of the antigen. Unfortunately, application of this approach to the treatment of autoimmune disease has been mostly unsuccessful in human clinical trials.7-11 One of the limitations of these tolerance-inducing strategies is that they do not ensure that the antigen is targeted to pathways that can promote tolerance, and, more importantly, to pathways that induce antigen-specific Tregs.12,13 This is partly because strategies for reliably targeting an antigen into these pathways in vivo have not been available, and these pathways are still poorly understood, making them difficult to target.14,15
An alternative to protein-based antigen administration is to use gene delivery as a means of inducing tolerance.16,17 Success has been achieved in animal models of autoimmunity and allograft transplant, but the overall effectiveness of gene-based approaches for inducing tolerance has been highly variable and limited.8,18,19 Indeed, in some cases, DNA vaccination has been found to exacerbate immunity to the gene-encoded antigen.20,21 This is not unexpected, as it is well established that gene delivery can prime an adaptive immune response.22 Our own attempts to correct hemophilia B in mice by injecting a lentiviral vector (LV) encoding coagulation factor IX (FIX) resulted in priming of an anti-FIX immune response and rapid clearance of gene-modified cells.23 Similar findings have been reported by many groups and for a wide range of applications and vector platforms.24-28
The induction of a cellular immune response against an antigen is the result of antigen presentation by professional antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs).29 APCs can present endogenously expressed or exogenously acquired antigens on major histocompatibility complex (MHC) class I molecules, and prime naive CD8+ T cells, which, in turn, target the destruction of cells expressing the antigen. As a means of preventing immune-mediated clearance of transduced cells, we recently developed a strategy to avoid expression of a gene-encoded construct in APCs.30 To do this, we exploited the endogenous microRNA (miRNA) regulatory network of APCs that are used to suppress gene expression in these cells. miRNAs are an abundant class of small noncoding RNAs found in all cell types that regulate gene expression by binding to cellular transcripts to suppress gene expression. By modifying a transgene to include target sequences for miR-142-3p (miR-142), a miRNA exclusively expressed in hematopoietic lineage cells,31 we accomplished 2 things, as follows: (1) expression of the transgene was suppressed in APCs, and (2) expression of transgene was maintained in nonhematopoietic cells where the miRNA was not expressed. This strategy enabled stable delivery of genes encoding green fluorescent protein (GFP), as well as FIX, in immunocompetent mice without signs of an immune response.23
In this study, we set out to determine whether a strategy to detarget antigen expression from APCs, by introducing miR-142 regulation into a LV, can subvert a pathogenic immune response and induce immunologic tolerance to the gene-encoded antigen. Our studies indicate that inhibiting direct antigen expression in APCs does not prevent T-cell priming, but does skew the direction of the immune response by causing rapid contraction of antigen-specific CD8+ T cells, and de novo induction of antigen-specific Tregs. Moreover, we provide data suggesting that this outcome is dependent on the antigen being expressed in hepatocytes. These studies have important implications for strategies aimed at inducing immunologic tolerance, and for understanding how cellular expression patterns dictate the outcome of an immune response.
Methods
Vector construction and animal procedures
Construction of pCCLsin.cPPT.PGK.GFP.wpre.142-3pT, pCCLsin.ET.GFP.wpre142-3pT, and pCCLsin.cPPT.PGK.GFP.wpre.142-3pT-122aT has been previously described.23,30,32 Vector administration was carried out by tail vein injection on 6- to 8-week-old mice.
DNA vaccination was performed, as previously described.33 Briefly, 0.5 nmol cardiotoxin-1 (Sigma-Aldrich) was injected in triadic leg muscles. Five days later, mice were injected again in the same position with 50 μg/leg pCCLsin.cPPT.CMV.GFP.wpre plasmid or pCCLsin.cPPT.CMV.HBs.wpre. Mice were euthanized 12 days after DNA administration.
All of the animal procedures were performed according to protocols approved by Hospital San Raffaele Institutional Animal Care and Use Committee (IACUC 321).
Interferon-γ enzyme-linked immunospot assay
Interferon-γ (IFN-γ)–secreting cells were enumerated by enzyme-linked immunospot (ELISPOT) assay in response to GFP-expressing cells. Briefly, 5 × 104 isolated splenic CD8+ T cells were plated in ELISPOT plates (Millipore) coated with anti-IFN-γ capture monoclonal antibody (mAb; 2.5 μg/mL, R46A2; BD Biosciences) in the presence of interleukin-2 (50 U/mL; BD Biosciences) and 5 × 104 irradiated (30 Gy) wild-type P815 or GFP+P815 cells. After 42 hours of incubation at 37°C/5% CO2, the plates were washed, and IFN-γ–producing cells were detected by anti-IFN-γ detection mAb (0.5 μg/mL, XMG 1.2; BD Biosciences). Spots were counted by ELI.Expert.Elispot-Reader and analyzed by Eli.Analyze software Version 5.1 (A.EL.VIS).
In vivo and in vitro cytotoxic T-lymphocyte assay
Twelve days postvaccination in vivo antigen-specific cytolytic activity was evaluated cotransferring the following: 5 × 106 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE)hi (2.5μM; Molecular Probes) GFP200-208 pulsed, 5 × 106 CFSEint (250nM) hepatitis B virus envelope small subunit immunodominant epitope (HBs)28-39 pulsed, and 5 × 106 CFSElow (25nM) unpulsed per mouse. Eighteen hours later, single-cell suspension was prepared from lymph nodes draining the site of vaccination. Ag-specific cytolysis was assayed by fluorescence-activated cell sorter (FACS) analysis acquiring a defined number of events for each sample and was calculated as 100−[(% vacc-pulsed/% vacc-UNpulsed)/(% UNvacc-pulsed/% UNvacc UNpulsed) × 100].
Splenic and liver-derived CD8+ T-cell cytotoxicity against GFP+P815 or wild type, as control, was evaluated in a standard 4-hour 51Cr release assay.
Statistical analysis
All statistical analyses were performed using the 2-tailed Student t test.
Results
Evidence of a modulated immune response to a miR-142–regulated antigen
We first set out to determine the immune response to an antigen regulated by miR-142. BALB/c mice were treated by intravenous injection with a LV encoding GFP and the target sequences for miR-142 in the 3′-untranslated region (LV.PGK.GFP.miR142-3pT [PGK.142T]). Transcription of the transgene was under the control of the ubiquitously expressed phosphoglycerokinase (PGK) promoter. As we previously reported, the control vector LV.PGK.GFP (PGK), which expresses GFP in all cell types transduced, including Kupffer cells, hepatocytes, and liver sinusoidal endothelial cells (LSECs), as well as a high frequency of MHC class II+ cells in the spleen,34 was completely cleared from the liver by 6 weeks postinjection. In contrast, PGK.142T, which does not express in hematopoietic cells, was able to mediate long-term gene transfer and expression of GFP in the liver (Figure. 1A), as well as a small population of nonhematopoietic stroma cells located specifically within the marginal zone sinus of the spleen.30 Quantitative polymerase chain reaction analysis showed that the vector copies/genome (C/G) in the liver of PGK.142T-treated mice was maintained over time (data not shown).
Monitoring the transgene-specific immune response in mice injected with PGK.142T. BALB/c mice were intravenously injected with LV PGK or PGK.142T encoding GFP. (A) Confocal fluorescent microscopy analysis showing GFP (green) expression patterns in the liver at the indicated times. Scale bar = 100 μm. (B) The frequency of IFN-γ–producing, GFP-specific CD8+ T cells in the spleen of treated mice was determined by ELISPOT at the indicated times. Data are expressed as the mean ± SD number of GFP-specific CD8+ T cells per 106 total CD8+ T cells. Measurement of the GFP+ cell-specific killing capacity of CTL derived from treated mice was performed by chromium release assay using different ratios of CD8+ effector T cells: P815 target cell line (P815 cell line, BALB/c syngeneic mastocytoma-derived cell line, was in vitro transduced to stably express GFP, or untransduced as control). (C) Measurement of the GFP-driven killing capacity of liver-derived CD8+ T cell; 10:1 ratio is reported. (D) Measurement of the GFP+ cell-specific killing capacity of spleen CD8+ T cell. The 100:1 ratio of effector:target cells is shown. Data are expressed as the mean ± SD percentage of target cell lysis. (E-F) The frequency of GFP-specific CD8+ T cells in the liver (E) and spleen (F) was determined by FACS analysis of intrahepatic leukocytes and splenocytes, respectively, stained with a pentamer loaded with the H-2Kd immunodominant epitope of GFP, GFP200-208. Analysis was performed 6 weeks postinjection (n = 3/group). A representative dot plot is shown. Below, the mean ± SD of the experiment is provided. (G) Quantification of the absolute number of CD8+ T cells infiltrating the liver 6 weeks after LV injection. Shown as the mean ± SD (n = 3/group).
Monitoring the transgene-specific immune response in mice injected with PGK.142T. BALB/c mice were intravenously injected with LV PGK or PGK.142T encoding GFP. (A) Confocal fluorescent microscopy analysis showing GFP (green) expression patterns in the liver at the indicated times. Scale bar = 100 μm. (B) The frequency of IFN-γ–producing, GFP-specific CD8+ T cells in the spleen of treated mice was determined by ELISPOT at the indicated times. Data are expressed as the mean ± SD number of GFP-specific CD8+ T cells per 106 total CD8+ T cells. Measurement of the GFP+ cell-specific killing capacity of CTL derived from treated mice was performed by chromium release assay using different ratios of CD8+ effector T cells: P815 target cell line (P815 cell line, BALB/c syngeneic mastocytoma-derived cell line, was in vitro transduced to stably express GFP, or untransduced as control). (C) Measurement of the GFP-driven killing capacity of liver-derived CD8+ T cell; 10:1 ratio is reported. (D) Measurement of the GFP+ cell-specific killing capacity of spleen CD8+ T cell. The 100:1 ratio of effector:target cells is shown. Data are expressed as the mean ± SD percentage of target cell lysis. (E-F) The frequency of GFP-specific CD8+ T cells in the liver (E) and spleen (F) was determined by FACS analysis of intrahepatic leukocytes and splenocytes, respectively, stained with a pentamer loaded with the H-2Kd immunodominant epitope of GFP, GFP200-208. Analysis was performed 6 weeks postinjection (n = 3/group). A representative dot plot is shown. Below, the mean ± SD of the experiment is provided. (G) Quantification of the absolute number of CD8+ T cells infiltrating the liver 6 weeks after LV injection. Shown as the mean ± SD (n = 3/group).
To monitor the immune response to the vector-encoded antigen, we examined the frequency of GFP-specific CD8+ T cells in the spleen and liver. Unexpectedly, despite the striking differences in the persistence of gene expression between the 2 treatment groups, we observed a similar induction of GFP-specific, IFN-γ–producing CD8+ T cells in an ELISPOT assay performed on spleen cells 1 week after vector injection. However, by 3 weeks postinjection, differences in the immune response between the 2 treatment groups became apparent. In PGK.142T vector-treated mice, there was a drastic contraction in the frequency of GFP-specific CD8+ T cells, whereas in mice treated with the PGK vector, the GFP-specific T cells persisted at higher levels (Figure 1B) even though antigen-expressing cells (ie, GFP expressing) had been cleared. In addition, assessment of GFP-specific cytotoxic activity indicated a strong reduction in the frequency of cytotoxic T lymphocytes (CTL) in PGK.142T-treated mice compared with PGK-treated mice. This finding was particularly striking in the spleen of PGK.142T-treated mice that displayed a contraction of the anti-GFP CTL by 3 weeks postinjection (29% vs 3% GFP-specific lysis activity from CD8+ splenic T cells from PGK vs PGK.142T vector-treated mice at 3 weeks).
To distinguish whether the loss of GFP-specific CD8+ T cells observed in the ELISPOT and lytic assay was due to a change in the functionality of the cells (ie, they underwent anergy) or due to elimination of the cells by clonal deletion, we analyzed the frequency of GFP-specific CD8+ T cells by staining with an H-2Kd pentamer loaded with the immunodominant epitope of GFP (GFP200-208). The data showed a significantly lower frequency of antigen-specific CD8+ T cells in both the liver and spleen of PGK.142T vector-treated mice (Figure 1E-F), although the absolute number of CD8+ T cells infiltrating the liver in PGK.142T-treated mice was not significantly different compared with PGK-treated mice (Figure 1G).
To better understand the events preceding the contraction of the CD8+ T cell–mediated response, we examined proliferation and expression of activation markers by GFP-specific CD8+ T cells at 2 weeks after LV injection. miR-142 regulation of antigen expression led to an increased proportion of central memory (CD8+GFP200-208-H2-Kd+CD44+CD62L+) and decreased rate of proliferation in secondary lymphoid organs of PGK.142T-treated mice compared with control PGK-treated mice (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results indicate that avoiding antigen expression in hematopoietic lineage cells via miR-142 regulation does not result in immunologic ignorance of the transgene-encoded antigen. Instead, miR-142 mediates a modified response to the vector-encoded antigen in which functional antigen-specific CTL are briefly expanded, followed by a rapid contraction, without resulting in clearance of the cells expressing the antigen.
miR-142–regulated gene transfer induces a robust state of antigen-specific tolerance
To investigate whether the administration of PGK.142T can establish immunologic tolerance to the transgene-encoded antigen, mice were treated with phosphate-buffered saline (PBS), PGK, or PGK.142T. Six weeks later, the mice were vaccinated by injection of a GFP-encoding plasmid into cardiotoxin-injured muscle. In PBS-treated mice, vaccination led to an increase in anti-GFP, IFN-γ–producing splenic CD8+ T cells, indicating that DNA vaccination alone was capable of inducing a primary cellular immune response to GFP (Figure 2A). In mice previously treated with the PGK vector, DNA vaccination resulted in a significant expansion of GFP-specific CD8+ T cells in the spleen, consistent with a secondary response (Figure 2A). The expansion of GFP-specific CD8+ T cells was milder, but detectable, in the liver (Figure 2B) most likely because the organ lacked antigen-expressing cells, as a result of prior immune-mediated clearance (Figure 1A).
Vaccine challenge of PGK.142T-treated mice. PGK- and PGK.142T-treated BALB/c mice were vaccinated at 6 weeks after vector injection by administration of a plasmid encoding GFP into cardiotoxin-injured muscle. Analysis was performed 12 days after vaccination. (A-B) The frequency of IFN-γ–producing GFP-specific CD8+ T cells in the spleen and liver of treated mice was determined by ELISPOT. Data are expressed as the mean ± SD number of GFP-specific CD8+ T cells per 106 total CD8+ T cells. Note that vaccination was able to induce a primary immune response to GFP in PBS-treated mice, and a secondary immune response to GFP in PGK-treated mice, but not in PGK.142T-treated mice. (C) Liver sections were analyzed by confocal microscopy after staining for GFP and nuclei. Scale bar = 100 μm. Images are representative of 2 separate experiments (n = 6 mice/group). Also shown is the mean C/G from the livers of treated mice (n = 6).
Vaccine challenge of PGK.142T-treated mice. PGK- and PGK.142T-treated BALB/c mice were vaccinated at 6 weeks after vector injection by administration of a plasmid encoding GFP into cardiotoxin-injured muscle. Analysis was performed 12 days after vaccination. (A-B) The frequency of IFN-γ–producing GFP-specific CD8+ T cells in the spleen and liver of treated mice was determined by ELISPOT. Data are expressed as the mean ± SD number of GFP-specific CD8+ T cells per 106 total CD8+ T cells. Note that vaccination was able to induce a primary immune response to GFP in PBS-treated mice, and a secondary immune response to GFP in PGK-treated mice, but not in PGK.142T-treated mice. (C) Liver sections were analyzed by confocal microscopy after staining for GFP and nuclei. Scale bar = 100 μm. Images are representative of 2 separate experiments (n = 6 mice/group). Also shown is the mean C/G from the livers of treated mice (n = 6).
In contrast to naive and PGK-treated mice, PGK.142T-treated mice did not respond to DNA vaccination. There was no detectable expansion of GFP-specific, IFN-γ–producing CD8+ T in either the spleen (Figure 2A) or the liver (Figure 2B), where the antigen was still highly expressed, and no increase in the overall number of CD8+ T cells in the liver upon vaccination (data not shown). The absence of an immune response to GFP in these mice after vaccination was confirmed by molecular and histologic analysis of the liver (Figure 2C). The frequency of cells expressing the transgene-derived antigen, and the number of C/G in vaccinated and unvaccinated mice were similar (0.75 ± 0.54 and 0.93 ± 0.49 C/G, respectively), indicating that there was no clearance of transduced cells after vaccination. Thus, these studies demonstrate that in addition to circumventing the primary immune response, miR-142 regulation of gene-encoded antigens, at least in the context of LV-mediated gene transfer, mediates a long-lasting and robust state of immunologic tolerance that cannot be broken by rechallenge with the antigen.
CD4+CD25+Foxp3+ Tregs are necessary for mediating stable miR-142–regulated gene transfer
Our analysis of the immune response to the miR-142–regulated transgene-encoded antigen indicated that there was an initial induction of lytic anti-GFP CD8+ T cells; however, GFP-expressing cells were not eliminated even after vaccination. This finding suggested that there may be active suppression of the anti-GFP response. To investigate whether this state of tolerance was mediated by Tregs, we examined the content of CD4+CD25+CTLA4+Foxp3+ Tregs in the liver of mice after treatment with PGK or PGK.142T vectors. At 3 weeks after vector injection, when the cellular immune response is clearly down-regulated (see Figure 1B-D), there was a 2-fold increase in the frequency of Tregs in the livers of mice injected with the PGK.142T vector (7.74% ± 0.2% in PGK.142T vs 4.42% ± 0.70% in PGK; Figure 3A-B). Conversely, in mice treated with PGK vector, the frequency of Tregs was the same as in PBS-treated control mice (4.42% ± 0.70% vs 3.47% ± 1.25%). Relative quantification of Foxp3 mRNA confirmed that there was an up-regulation of Foxp3 expression in CD4+ T cells isolated from the liver of PGK.142T vector-treated mice, but not PGK-treated mice (data not shown). To discriminate whether the increase of Tregs was due to an expansion or an accumulation, we stained the CD4+CD25+Foxp3+ cells in the liver with Ki67, which detects cell proliferation (supplemental Figure 2). Our results indicate that the Treg proliferation rate was comparable in PGK- and vehicle-treated mice, whereas the proliferation rate was increased in mice treated with the miR-142–regulated LV. Thus, the increase in Tregs observed in the liver of PGK.142T-treated mice was due to a de novo expansion in the tissue.
Measurement of CD4+CD25+Foxp3+ Tregs in the liver of PGK and PGK.142T vector-treated mice. Mice were injected with vehicle, PGK, or PGK.142T. (A) At the indicated times, mice were killed and the frequency of CD4+CD25+Foxp3+ Tregs in the liver was determined by FACS analysis. Data are expressed as the mean ± SD. A representative 3 experiments is shown, n = 3/group (n = 9/vector), except for vehicle injected (n = 17). (B) Representative dot plot gated on CD4+ cells is presented for each group at 3 weeks postinjection. The percentage of CD4+CD25+Foxp3+ Tregs is reported in the top right quadrant. CTLA-4 expression is shown gating on CD4+CD25+Foxp3+ Tregs or CD4+Foxp3− T cells.
Measurement of CD4+CD25+Foxp3+ Tregs in the liver of PGK and PGK.142T vector-treated mice. Mice were injected with vehicle, PGK, or PGK.142T. (A) At the indicated times, mice were killed and the frequency of CD4+CD25+Foxp3+ Tregs in the liver was determined by FACS analysis. Data are expressed as the mean ± SD. A representative 3 experiments is shown, n = 3/group (n = 9/vector), except for vehicle injected (n = 17). (B) Representative dot plot gated on CD4+ cells is presented for each group at 3 weeks postinjection. The percentage of CD4+CD25+Foxp3+ Tregs is reported in the top right quadrant. CTLA-4 expression is shown gating on CD4+CD25+Foxp3+ Tregs or CD4+Foxp3− T cells.
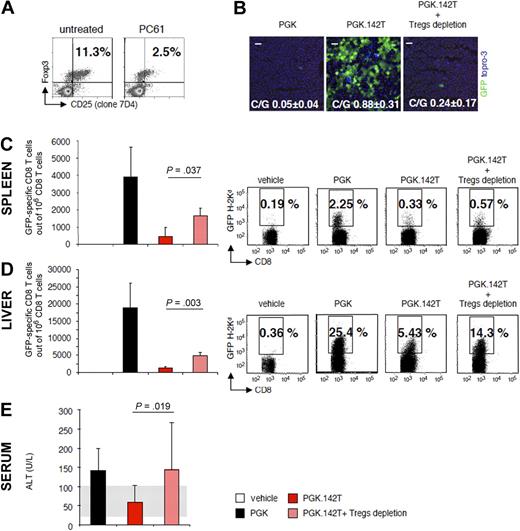
To elucidate whether the increased frequency of Tregs was functionally related to the establishment of immunologic tolerance to antigen-expressing cells, we monitored the effect of depleting Tregs on PGK.142T-mediated gene transfer. Mice were treated with the PC61 mAb (anti-CD25 mAb), and the reduced frequency of CD4+CD25int/bright cells, which correspond to naturally occurring Tregs, was confirmed by FACS analysis (Figure 4A). In Treg-depleted mice receiving the PGK.142T vector, there were virtually no GFP-expressing cells in the liver at 6 weeks after injection (Figure 4B), and quantification of vector genomes by quantitative polymerase chain reaction found that there was a significant reduction in vector content in depleted versus nondepleted mice receiving PGK.142T (0.24 ± 0.17 vs 0.88 ± 0.31 C/G, respectively; note that we expect to detect vector genomes in the absence of GFP expression in the liver of these mice, as a good fraction of the injected vector ends up in Kupffer cells, where it is not expressed, due to miR-142 regulation, and thus, it is not subjected to immune-mediated clearance). Concomitant with the clearance of antigen-expressing cells in Treg-depleted mice, we detected an expansion of GFP-specific, IFN-γ–producing CD8+ T cells in both the liver and spleen (Figure 4C-D), demonstrating that the loss of GFP expression was a result of immune-mediated clearance. Moreover, alanine liver transaminase (ALT) levels, representing an indirect measure of hepatic damage, were elevated in the sera of Treg-depleted PGK.142T-treated mice, and comparable with the values detected in the sera of PGK-treated control mice (Figure 4E). Thus, our findings indicate that Tregs are necessary for establishing stable gene transfer and expression of an antigen in the liver.
Examination of the role and function of CD4+CD25+Foxp3+ Tregs in PGK.142T-mediated gene transfer. Mice were injected with the PC61 mAb (anti–mouse CD25, 1 mg/mouse) 5 days before treatment with PGK.142T. (A) At the time of vector injection, the number of Tregs was determined by FACS analysis of splenocytes immunostained for CD4, CD25 (clone 7D4), and Foxp3. (B) At 6 weeks after vector injection, mice were killed and GFP expression in the liver was monitored by confocal microscopy. GFP was visualized by anti-GFP staining (green), and nuclei by Topro-3 staining (blue). Also shown is the mean ± SD C/G from the livers of treated mice (n = 9). (C-D) The frequency of IFN-γ–producing, GFP-specific CD8+ T cells in the spleen (C) and liver (D) of treated mice was determined by ELISPOT assay. Data are expressed as the mean ± SD number of GFP-specific CD8+ T cells per 106 total CD8+ T cells. For the spleen, analysis was performed on isolated CD8+ T cells, whereas for the liver, analysis was performed on total intrahepatic leukocytes, and the amount of CD8+ T cells was determined by FACS analysis (data not shown). The frequency of GFP-specific CD8+ T cells in the spleen (C) and liver (D) was determined by FACS analysis of splenocytes and intrahepatic leukocytes, respectively, stained with a H-2Kd pentamer loaded with the immunodominant epitope of GFP, HYLSTQSAL (GFP200-208). A representative dot plot is shown from 1 of 3 separate experiments (n = 3/group per experiment; n = 9/vector). (E) Transaminase levels (ALT U/L) were determined in the sera of treated mice at the time of sacrifice; data are expressed as mean ± SD (n = 6/group). Normal ALT levels, detected in the sera of vehicle-injected mice, are 60 ± 40 U/L (n = 3, gray area).
Examination of the role and function of CD4+CD25+Foxp3+ Tregs in PGK.142T-mediated gene transfer. Mice were injected with the PC61 mAb (anti–mouse CD25, 1 mg/mouse) 5 days before treatment with PGK.142T. (A) At the time of vector injection, the number of Tregs was determined by FACS analysis of splenocytes immunostained for CD4, CD25 (clone 7D4), and Foxp3. (B) At 6 weeks after vector injection, mice were killed and GFP expression in the liver was monitored by confocal microscopy. GFP was visualized by anti-GFP staining (green), and nuclei by Topro-3 staining (blue). Also shown is the mean ± SD C/G from the livers of treated mice (n = 9). (C-D) The frequency of IFN-γ–producing, GFP-specific CD8+ T cells in the spleen (C) and liver (D) of treated mice was determined by ELISPOT assay. Data are expressed as the mean ± SD number of GFP-specific CD8+ T cells per 106 total CD8+ T cells. For the spleen, analysis was performed on isolated CD8+ T cells, whereas for the liver, analysis was performed on total intrahepatic leukocytes, and the amount of CD8+ T cells was determined by FACS analysis (data not shown). The frequency of GFP-specific CD8+ T cells in the spleen (C) and liver (D) was determined by FACS analysis of splenocytes and intrahepatic leukocytes, respectively, stained with a H-2Kd pentamer loaded with the immunodominant epitope of GFP, HYLSTQSAL (GFP200-208). A representative dot plot is shown from 1 of 3 separate experiments (n = 3/group per experiment; n = 9/vector). (E) Transaminase levels (ALT U/L) were determined in the sera of treated mice at the time of sacrifice; data are expressed as mean ± SD (n = 6/group). Normal ALT levels, detected in the sera of vehicle-injected mice, are 60 ± 40 U/L (n = 3, gray area).
miR-142–regulated gene transfer induces antigen-specific Tregs
Although our data indicate that Tregs are involved in establishing stable gene transfer, it is unclear whether the increased frequency of Tregs observed was specific for the gene-encoded antigen, or represented natural Tregs (nTregs) that had homed to the liver to subdue the immune response. Whereas there is evidence that specific Tregs for a foreign antigen can be induced,35,36 this has not been shown to occur with a strong xenoantigen expressed intracellularly, and absent in the educational process of the thymus.16
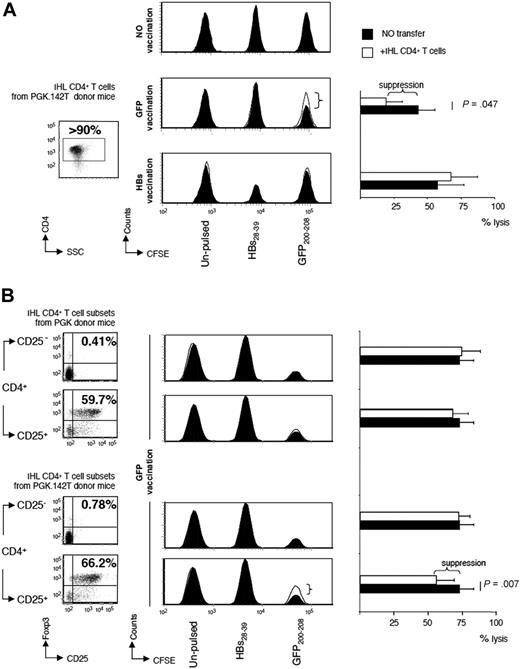
To address the issue of antigen specificity, we isolated CD4+ T cells from the liver of PGK.142T vector-treated mice, and adoptively transferred them into naive BALB/c mice (white histogram). A control group of BALB/c mice did not receive adoptive transfer of any cells (black histogram). Two days later, the mice were vaccinated with a plasmid encoding either GFP or the small protein of HB envelope. At 12 days after vaccination, in vivo CTL assay was performed. Recipient mice either were injected with splenocytes pulsed with the immunodominant epiptope of GFP, GFP200-208, and labeled with 2.5μM CFSE (GFP200-208/CFSEhi), pulsed with the immunodominant epiptope of hepatitis B virus env, HBs28-39, and labeled with 250nM CFSE (HBs28-39/CFSEint), or left unpulsed and labeled with 25nM CFSE (CFSElow). By monitoring the number of CFSEhi cells (presenting GFP200-208), CFSEint cells (presenting HBs28-39), and CFSElow cells (presenting self-antigens) in the draining lymph nodes of the site of vaccination of recipient mice, we found that there is CTL-mediated lysis of the CFSE-labeled target cells after vaccination.
In mice that received no vaccinations, there was no difference in the number of CFSEhi, CFSEint, and CFSElow cells, indicating that the labeled cells are stable for the observation time in the absence of vaccination (Figure 5A). However, mice vaccinated with the plasmid encoding HBs had a loss of CFSEint cells, but not CFSEhi cells or CFSElow cells, demonstrating that a HBs-specific immune response had been induced. There was no difference in the residual number of CFSEint cells between HBs-vaccinated mice receiving CD4+ T cells and those that did not (Figure 5A bottom histograms). This indicates that the transferred CD4+ T cells did not contain a population that is capable of suppressing the anti-HBs immune response induced by vaccination. Instead, in mice that were vaccinated against GFP, there was a significantly higher loss in CFSEhi (presenting cells GFP200-208) when they did not receive adoptive transfer of CD4+ T cells (black histogram), compared with mice that did receive CD4+ T cells from PGK.142T-treated mice (white histogram), indicating that the anti-GFP CTL response had been subdued by the CD4+ T cells from PGK.142T vector-treated mice (Figure 5A middle histograms). These results indicate that liver-infiltrating CD4+ T cells isolated from PGK.142T-treated mice comprise a transgene-specific regulatory CD4+ T-cell subpopulation.
Evaluation of antigen specificity of CD4+CD25+Foxp3+ Tregs in the liver of PGK.142T-treated mice. (A) CD4+ T cells were isolated from the livers of mice treated 3 weeks prior with PGK.142T. Cell purity was determined by FACS analysis (> 90%). Purified hepatic CD4+ T cells from PGK.142T-treated mice were injected into naive recipient mice (106 CD4+ T cells/mouse). Two days later, the mice received DNA vaccination to induce a cellular immune response to GFP or to hepatitis B small env subunit (HBs), an unrelated antigen. Twelve days after DNA vaccination, in vivo CTL assay was performed. Mice were infused with splenocytes labeled with different concentrations of CFSE and pulsed with either HBs28-39 (CFSEint) or GFP200-208 (CFSEhi), or left unpulsed (CFSElow). Draining lymph nodes were collected, and FACS analysis was performed to quantitate the surviving cells. Mice receiving vaccination alone are show as filled histograms (n = 5), and mice receiving vaccination plus adoptive transfer of hepatic CD4+ T cells are shown as open histograms (n = 3). A representative histogram for each experimental group is shown (no vaccination, top panel; GFP vaccinated, midpanel; HBs vaccinated, bottom panel). The mean percentage ± SD of antigen-driven target cell lysis values for each experimental group of mice is reported (A right panel). (B) Liver-infiltrating CD4+ T cells isolated from mice treated with PGK or tolerized to GFP by PGK.142T vector were sorted by CD25 expression, and the 4 resulting populations were adoptively transferred (0.8 × 105 cell/mouse) into naive mice that underwent GFP vaccination 2 days later. As described above, in vivo CTL assay was performed 12 days after vaccination. Mice receiving vaccination alone are shown as filled histograms (n = 10), and mice receiving vaccination plus adoptive transfer of hepatic CD4+CD25− (n = 8) and CD4+CD25+ (n = 8) T cells derived either from PGK- or PGK.142T-treated mice are shown as open histograms. A representative histogram for each experimental group is shown. The mean percentage of antigen-driven target cell lysis ± SD values for each experimental group of mice is reported (B, right panel).
Evaluation of antigen specificity of CD4+CD25+Foxp3+ Tregs in the liver of PGK.142T-treated mice. (A) CD4+ T cells were isolated from the livers of mice treated 3 weeks prior with PGK.142T. Cell purity was determined by FACS analysis (> 90%). Purified hepatic CD4+ T cells from PGK.142T-treated mice were injected into naive recipient mice (106 CD4+ T cells/mouse). Two days later, the mice received DNA vaccination to induce a cellular immune response to GFP or to hepatitis B small env subunit (HBs), an unrelated antigen. Twelve days after DNA vaccination, in vivo CTL assay was performed. Mice were infused with splenocytes labeled with different concentrations of CFSE and pulsed with either HBs28-39 (CFSEint) or GFP200-208 (CFSEhi), or left unpulsed (CFSElow). Draining lymph nodes were collected, and FACS analysis was performed to quantitate the surviving cells. Mice receiving vaccination alone are show as filled histograms (n = 5), and mice receiving vaccination plus adoptive transfer of hepatic CD4+ T cells are shown as open histograms (n = 3). A representative histogram for each experimental group is shown (no vaccination, top panel; GFP vaccinated, midpanel; HBs vaccinated, bottom panel). The mean percentage ± SD of antigen-driven target cell lysis values for each experimental group of mice is reported (A right panel). (B) Liver-infiltrating CD4+ T cells isolated from mice treated with PGK or tolerized to GFP by PGK.142T vector were sorted by CD25 expression, and the 4 resulting populations were adoptively transferred (0.8 × 105 cell/mouse) into naive mice that underwent GFP vaccination 2 days later. As described above, in vivo CTL assay was performed 12 days after vaccination. Mice receiving vaccination alone are shown as filled histograms (n = 10), and mice receiving vaccination plus adoptive transfer of hepatic CD4+CD25− (n = 8) and CD4+CD25+ (n = 8) T cells derived either from PGK- or PGK.142T-treated mice are shown as open histograms. A representative histogram for each experimental group is shown. The mean percentage of antigen-driven target cell lysis ± SD values for each experimental group of mice is reported (B, right panel).
Because PGK.142T treatment leads to a 2-fold increase in the frequency of liver-infiltrating CD4+CD25+Foxp3+T cells (Figure 3A), the entire liver-derived CD4+ T-cell populations isolated either from PGK- or PGK.142T-treated mice were sorted by the expression of CD25, and transferred into recipient mice 2 days before GFP vaccination. Twelve days after vaccination, in vivo CTL assay was performed by infusing the 3 populations of CFSE-labeled cells into untreated mice, in mice only GFP vaccinated, and, lastly, in 4 groups of mice that received the 4 resulting CD4+ T-cell subsets: CD4+CD25− or CD4+CD25+ T cells from PGK- or PGK.142T-injected mice (Figure 5B, dot plots on the left). GFP vaccination led to loss of CFSEhi cells, but not CFSEint cells or CFSElow cells, demonstrating that a GFP-specific immune response had been induced. There was no difference in the residual number of CFSEhi cells between GFP-vaccinated mice receiving CD4+CD25− or CD4+CD25+ T cells from PGK-injected mice or CD4+CD25− T cells from PGK.142T-injected mice and those that did not. On the contrary, anti-GFP CTL response was partially, but significantly (P = .007), suppressed by CD4+CD25+ T cells from PGK.142T-injected mice compared with controls (Figure 5B, bottom histograms).
These findings provide strong evidence that detargeting antigen expression from professional APCs via miR-142 regulation induces a population of antigen-specific Tregs even for an intracellular antigen, with little or no expected homology to self-antigens.
Antigen expression in hepatocytes is required to prevent immune-mediated clearance of transduced cells
Intravenous injection of vesicular stomatitis virus–pseudotyped LV results predominately in transduction of professional APCs, which include DCs, Kupffer cells, hepatocytes, and LSECs in the liver, and a high frequency of MHC class II+ cells in the spleen. Our studies indicate that preventing antigen expression in professional APCs by miR-142 regulation results in induction of antigen-specific Tregs and tolerance to the vector-encoded antigen. It remains unclear, however, what role other transduced cells play in mediating tolerance.
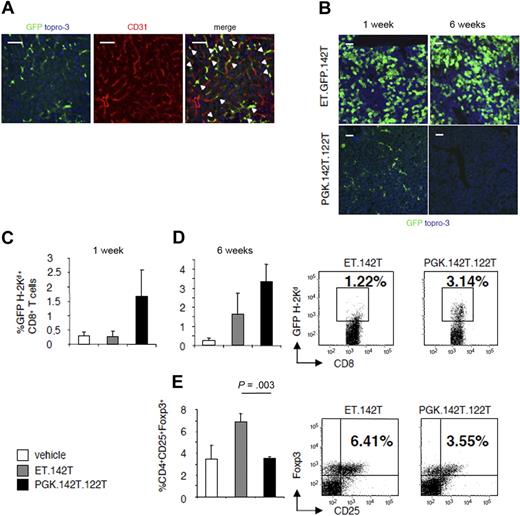
To address this issue, we evaluated the immune response to GFP using vectors that selectively target transgene expression to either LSECs or hepatocytes. For targeting vector expression to hepatocytes, we substituted the PGK promoter in the miR-142–regulated vector with the hepatocyte-specific (ET) enhanced transthyretin promoter (ET.GFP.142T). ET.142T mediates high levels of transgene expression in hepatocytes, without off-target expression in hematopoietic lineage cells.23 To target antigen expression to LSECs, we modified the PGK.142T vector to contain 4 perfectly complementary target sites for the hepatocyte-specific miRNA, miR-122a (PGK.142T.122T). Through this design, transgene expression is detargeted from both hematopoietic lineage cells and hepatocytes, and maintained in CD31+ LSECs (Figure 6A), and other transduced cells, which do not express either miR-142 or miR-122a.
Determination of the contribution of LSECs and hepatocytes to the stability of gene transfer. Mice were injected with either the hepatocyte-specific vector, ET.142T, or a LV-containing target sequence for miR-122 and miR-142, PGK.142T.122T. (A) Confocal fluorescent microscopy was performed on liver section to analyze the pattern of GFP expression (green). Sections were stained with anti-CD31 (red) to detect liver sinusoidal endothelial cells. Note that the majority of GFP-positive cells are also positive for CD31 (yellow), indicating that PGK.142T.122T expression is confined to endothelial cells. (B) Confocal fluorescent microscopy of the liver at 1 and 6 weeks after vector injection. GFP was visualized by anti-GFP staining, and nuclei by Topro-3 staining (blue). Scale bar = 100 μm. A representative image of 2 experiments (n = 3/group per experiment) is shown. (C-D) Leukocytes infiltrating the liver were isolated (C) 1 and (D) 6 weeks after vector administration, and the frequency of GFP-specific CD8+ T cells was evaluated by H-2Kd-GFP200-208 pentamer staining. Data are expressed as the mean ± SD percentage of GFP200-208-H2-Kd+CD8+ T cells. To the right, a representative dot plot, gated on CD8+ cells, is shown from 1 of 2 experiments (n = 3/group per experiment). (E) The frequency of CD4+CD25+Foxp3+ Tregs was quantified by FACS analysis. Data are expressed as mean ± SD percentage of positive cells. To the right, a representative dot plot, gated on CD4+ T cells, is shown from 1 of 2 experiments (n = 3/group per experiment).
Determination of the contribution of LSECs and hepatocytes to the stability of gene transfer. Mice were injected with either the hepatocyte-specific vector, ET.142T, or a LV-containing target sequence for miR-122 and miR-142, PGK.142T.122T. (A) Confocal fluorescent microscopy was performed on liver section to analyze the pattern of GFP expression (green). Sections were stained with anti-CD31 (red) to detect liver sinusoidal endothelial cells. Note that the majority of GFP-positive cells are also positive for CD31 (yellow), indicating that PGK.142T.122T expression is confined to endothelial cells. (B) Confocal fluorescent microscopy of the liver at 1 and 6 weeks after vector injection. GFP was visualized by anti-GFP staining, and nuclei by Topro-3 staining (blue). Scale bar = 100 μm. A representative image of 2 experiments (n = 3/group per experiment) is shown. (C-D) Leukocytes infiltrating the liver were isolated (C) 1 and (D) 6 weeks after vector administration, and the frequency of GFP-specific CD8+ T cells was evaluated by H-2Kd-GFP200-208 pentamer staining. Data are expressed as the mean ± SD percentage of GFP200-208-H2-Kd+CD8+ T cells. To the right, a representative dot plot, gated on CD8+ cells, is shown from 1 of 2 experiments (n = 3/group per experiment). (E) The frequency of CD4+CD25+Foxp3+ Tregs was quantified by FACS analysis. Data are expressed as mean ± SD percentage of positive cells. To the right, a representative dot plot, gated on CD4+ T cells, is shown from 1 of 2 experiments (n = 3/group per experiment).
BALB/c mice were injected intravenously with PBS, ET.142T, or PGK.142T.122T. At 1 week and 6 weeks after injection, the mice were killed, and the persistence of transgene-expressing cells and the immune response to GFP were monitored. Gene transfer in PGK.142T.122T vector-treated mice was short lived. By 6 weeks after injection, we observed clearance of antigen-expressing cells (Figure 6B). Instead, in mice treated with ET.GFP.142T, gene transfer was stable, as indicated by the persistence of GFP-expressing hepatocytes at 6 weeks after injection.
Evaluation of the anti-GFP cellular immune response by pentamer staining of liver-infiltrating lymphocytes found that PGK.142T.122T provoked a greater expansion of GFP-specific CD8+ T cells than ET.GFP.142T, starting from 1 week after vector administration, indicating that CD8+ T cells were more efficiently primed in PGK.142T.122T vector-treated mice (Figure 6C-D). In addition, the frequency of Tregs was significantly higher in the liver of mice that received the vector expressing ET.142T compared with mice that received the PGK.142T.122T-expressing vector (6.9% ± 0.7% and 3.49% ± 0.16%, respectively; Figure 6E). Indeed, there was no evidence of increased CD4+CD25+Foxp3+ Tregs in the liver of PGK.142T.122T vector-treated mice.
Together these results demonstrate that detargeting antigen expression from professional APCs is insufficient to prevent an antigen-specific immune response, but, instead, there is an additional requirement for antigen to be specifically expressed by hepatocytes to achieve stable antigen expression in the liver and recruitment of Tregs.
Discussion
Our previous studies demonstrated that endogenous miR-142 regulation could be exploited to enable long-term transfer and expression of a transgene encoding a neoantigen in immunocompetent mice.23,30 In this study, we show that miR-142 regulation prevents immune-mediated clearance of transduced cells by the induction of antigen-specific Tregs to the transgene-encoded antigen. Our approach, which uses the miRNA regulatory network of hematopoietic cells to detarget expression of an antigen from APCs, provides a therapeutically relevant means for inducing antigen-specific immunologic tolerance.
We previously speculated that miR-142 enables stable gene transfer by limiting antigen presentation in professional APCs, and thereby preventing T-cell priming.30 The results reported in this study overturn this perspective. By examining the immune response at 1 week after injection, we found that there was a similar induction of GFP-specific CD8+ T cells in PGK.142T vector-treated mice as mice treated with the control vector that expresses GFP ubiquitously. T-cell priming in PGK.142T-treated mice was not due to a low level of antigen expression mediated by the vector in APCs, as adoptive transfer of PGK.142T-engineered DCs was unable to stimulate a GFP-specific CD8+ T-cell response (supplemental Figure 3). Interestingly, in PGK.142T vector-treated mice, the anti-GFP T cells did not mediate clearance of cells expressing GFP, and by 3 weeks after injection the frequency of anti-GFP T cells had substantially contracted. These results indicate that miR-142–mediated inhibition of transgene expression does not prevent T-cell priming by lentiviral delivery, but that in the absence of efficient antigen presentation by professional APCs, T cells are primed to a tolerogenic fate.
Although professional APCs, like Kupffer cells and DCs, are usually the source of T-cell priming during an immune response, in the liver, hepatocytes and LSECs also have the capacity to present antigens to naive T cells.37 LSECs express MHC I and II complexes, as well as the costimulatory molecules CD40, CD80, and CD86, and have been implicated in T-cell immunity and tolerance.38 Hepatocytes also express MHC I complexes at level comparable with splenocytes,39 as well as the costimulatory molecule, intercellular adhesion molecule-1.40 The ability of hepatocytes to prime naive CD8+ T cells directly in the liver, even in the absence of CD4+ T-cell help, has been shown in several transgenic models, as well as after gene transfer with adeno-associated viral vectors.41,42
Cross-presentation by APCs has also been considered as a possible source of priming, but the kinetics and strength of the response shown in our study would strongly argue against this possibility. In both the PGK and PGK.142T vector-treated mice, there was a similar induction of GFP-specific CD8+ T cells. This is quite striking because the former vector mediates high levels of antigen expression in APCs, whereas transgene expression from the PGK.142T vector is substantially inhibited in APCs. Because APCs' access to an intracellular antigen expressed by another cell should be a rare event, at least at early time points after vector administration, it is likely that the source of T-cell priming in mice treated with the PGK.142T vector is hepatocytes and/or LSECs in the liver.
The liver is known to be a site of tolerance induction, particularly in the context of organ transplantation. Due to the antigen-presenting capacity of LSECs, they have been implicated as the cellular source of the liver tolerogenic properties.43 Indeed, it has been shown that CD4+ T cells that are primed by LSECs differentiate toward an anti-inflammatory phenotype.38,44 In this study, using miRNA regulation to selectively detarget antigen expression from hematopoietic cells (142T), and hepatocytes (122T), and transcriptional targeting to eliminate expression in hematopoietic cells and LSECs (ET.142T), we determined that antigen expression in hematopoietic cells, nonhematopoietic, and nonhepatic cells leads to a productive immune response against vector-encoded antigens. Conversely, when antigen expression is preferentially targeted to hepatocyte, an altered immune response occurs that leads to the induction of antigen tolerance. Interestingly, it has been shown that naive T cells can be primed by hepatocytes in vitro and using transgenic models, but T cells die prematurely.45,46 This may be cell intrinsic, and related to how the CD8+ T cells are primed (presence of costimulation, etc), or because without APC priming, there is no CD4+ T-cell help for the CD8+ T cells. In either case, the initial CD8+ T-cell priming could lead to the type of early contraction we observed. Based on these findings, and our observations in this study, miR-142–regulated gene transfer is likely to be inducing tolerance, at least in part, by exploiting the antigen-presenting properties of hepatocytes.
miR-142–regulated gene transfer was able to establish a robust state of active immune tolerance to the vector-encoded antigen. This was evident by the persistence of GFP expression in the liver, even after DNA vaccination. Indeed, PGK.142T vector-treated mice did not show evidence of a secondary immune response after vaccination. Pentamer and ELISPOT analysis found a lower frequency of GFP-specific CD8+ T cells in the liver and spleen of PGK.142T vector-treated mice, suggesting that tolerance was achieved, at least in part, through T-cell deletion. This is consistent with the reported ability of hepatocytes to delete CD8+ T cells in an antigen-specific manner.45,46
In addition to a deletion mechanism, we also demonstrated that PGK.142T gene transfer induced Tregs specific for the transgene-encoded antigen, and displayed an nTreg-like phenotype. A potentially related finding was reported by Herzog and colleagues for AAV-mediated hepatic gene transfer.35 Using DO11.10-transgenic recombination-activating gene-2–/– mice, which are deficient for nTregs, they elegantly demonstrated that injection of an ovalbumin-encoding vector can induce ovalbumin-specific Tregs. Interestingly, a study by the High group found that nonhuman primates given daclizumab, an anti-interleukin-2 receptor antibody that works similar to the PC61 antibody, developed anti-FIX–neutralizing antibodies in response to treatment with an AAV vector encoding FIX, providing further evidence that Tregs (which are depleted by daclizumab) are required to maintain transgene expression in the liver.47 However, in these studies, along with those of Herzog, the transgene-encoded protein is secreted, and it was not known whether Tregs were being induced locally in the liver, or remotely, in an organ such as the thymus. In our model, we can rule out a role for the thymus because intravenous injection of LV does not transduce cells in the thymus, as indicated by histologic examination of GFP expression and molecular analysis of vector copies in the thymus (data not shown; vehicle [n = 2] 0.0024 ± 0.0002 C/G, PGK.142T [n = 5] 0.003 ± 0.0011 C/G), and GFP is an intracellular antigen, which would have little access to thymic APCs.
Several previous studies, including our own, have used hepatocyte-specific promoters in gene transfer vectors to avoid transgene expression in professional APCs. In many cases, this prevented an immune response to the transgene-encoded antigen, although when restriction to hepatocytes was not complete, induction of an immune response was observed in stringent models of immunity.28 The work presented in this study suggests that targeting expression to hepatocytes is important not only to avoid direct presentation by professional APCs, but also to specifically target expression to hepatocytes. Indeed, a recent attempt to use miR-142 target sites to prevent the immune response to an antigen encoded by a plasmid delivered to the liver by hydrodynamic injection failed when the ubiquitously expressed cytomegalovirus promoter was used, but not when a hepatocyte-specific promoter was used.48 This may be because expression of the target-bearing transgene from the cytomegalovirus promoter saturated miR-142 regulation, and antigen expression was not well suppressed in APCs.49 Because expression of the plasmid occurs within hours of injection,48 transgene-derived antigen would be presented in the context of inflammation induced by the plasmid, and this would lead to immunity.22
The actual mechanism by which hepatic gene transfer can induce or recruit Tregs and use them for preventing clearance of transduced cells remains unclear.16,35 Activation of Tregs requires MHC class II,50 which is not normally expressed by hepatocytes. Professional APCs in the liver may acquire transgene-derived antigen indirectly from hepatocytes, and present the antigens on MHC class II to CD4+ T cells. Importantly, this would be spatially and temporally separated from the initial vector transduction, and would mean that these professional APCs are likely to be in steady state, and can promote tolerance to the acquired antigen. This mechanism may not operate if there is prolonged or chronic inflammation, but under normal conditions it may be a way tolerance is generated to an antigen in the liver.
The ability to exploit miR-142 regulation, in the context of LV-mediated gene transfer, to induce immunologic tolerance to a gene-encoded antigen has relevant therapeutic implications. For the treatment of genetic diseases by LV-mediated gene replacement therapy, exploiting miR-142 regulation can ensure that gene transfer is stable so that long-term correction of the disease can be achieved. Moreover, because this approach can mediate deletion of antigen-reactive T cells, and induction of antigen-specific Tregs, it may be possible to exploit miR-142 regulation to enhance tolerance to autoantigens in people at risk of developing autoimmunity. Further studies in clinically relevant models of autoimmune disease will be necessary to evaluate this possibility. Nonetheless, these studies provide demonstration that miR-142 can be exploited to induce active immunologic tolerance to a transgene-encoded antigen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Angela Stabilini and Tatiana Jofra for technical assistance; Luca Guidotti, Kevin Goudy, and Manuela Battaglia for scientific discussions and editorial comments; and Giovanni Sitia, Andrea Valle, and Nicola Gagliani for scientific discussions.
This work was supported by Italian Telethon Foundation. B.D.B. was supported by the National Institutes of Health's Diabetes Pathfinder Award (DP2DK083052-01).
National Institutes of Health
Authorship
Contribution: A.A. and B.D.B. designed and performed experiments, collected and analyzed data, and wrote the paper; A.C. performed experiments; L.S.S. performed vector preparations; and L.N. and M.-G.R. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.D.B. is Department of Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY.
Correspondence: Maria-Grazia Roncarolo, San Raffaele Telethon Institute for Gene Therapy Via Olgettina 58, 20132 Milan, Italy; e-mail: m.roncarolo@hsr.it.
References
Author notes
A.A., B.D.B., L.N., and M.-G.R. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal