Abstract
Afferent lymph is transported throughout lymph nodes (LNs) by the conduit system. Whereas this conduit network is dense in the T-cell zone, it is sparse in B-cell follicles. In this study, we show that this differential organization emerges during lymph node development. Neonatal LNs lack B follicles, but have a developed T-cell zone and a dense conduit network. As new T and B cells enter the developing LN, the conduit network density is maintained in the T, but not the B zone, leading to a profound remodeling of the follicular network that nevertheless maintains its connectivity. In adults, the residual follicular conduits transport soluble antigen to deep regions, where follicular dendritic cells are abundant and appear to replace the fibroblastic reticular cells that enwrap conduits in the T zone. This strategic location correlates with the capacity of the follicular dendritic cells to capture antigen even in the absence of antigen-specific antibodies. Together, these results describe how the stromal organization of the T and B regions of LNs diverges during development, giving rise to distinct antigen transport and delivery modes in the 2 compartments.
Introduction
The development of an adaptive immune response takes place in secondary lymphoid organs such as spleen and lymph nodes (LNs). One of the most important functions of LNs is to continuously gather the soluble and cellular information conveyed by the lymphatics draining peripheral tissues.1 The afferent lymph is discharged into a superficial, volume-constrained region of the LN, the subcapsular sinus (SCS). How cells and soluble factors entering the SCS move across the floor of this structure and into the LN parenchyma is still debated. For many years, it was presumed that the floor of the SCS was porous and that soluble antigens (Ags), cytokines, and chemokines were able to freely diffuse into the underlying B-cell follicles.2-5 More recently, however, studies revealed the existence of a specialized transport system composed of conduits with a collagen fiber core surrounded by fibroblastic reticular cells (FRCs), a subset of myofibroblasts.6,7 Gretz et al showed that these conduits primarily transported molecules smaller than 50 to 70 kDa.8-10 Using these “pipes,” chemokines and soluble Ags are rapidly transported to high endothelial venules (HEVs) and to the dendritic cells (DCs) firmly attached to this network.10-12
In contrast to the rich, connected conduit network in the T zone (paracortex), this “information highway” is very sparse in the B-cell follicles, raising questions about both what gives rise to the very different organization of these stromal structures in the T versus B areas of LNs and what function the smaller number of conduits might have in B follicles.6,13 Roozendaal et al recently revealed one function of this follicular network, showing that the conduit system rapidly delivers small Ags present in subcutaneous tissues to follicular B cells.13 These data on conduit delivery of small soluble Ags from the skin provide an alternative explanation for what was considered to be the free diffusional entry of such substances into the follicle from the SCS, as reported by Pape et al.14
Recent studies by other laboratories have highlighted the role of myeloid cells in Ag delivery to naive B cells. For example, DCs originating from inflamed peripheral tissues migrate to the draining LNs via the lymphatics, enter the paracortex in the interfollicular region where the FRCs abut the SCS, and eventually migrate to the T-cell zone.15 Upon their arrival in the paracortex, DCs preferentially settle close to HEVs, the entry site of blood lymphocytes into the LN,16 allowing these DCs to rapidly activate the T and B cells that recently entered the LN via these vessels.16,17 Because HEVs are present in the T-cell zone, recently emigrant B cells have to transit the T-cell zone before they reach the follicle. We have shown that, like T cells, B cells migrate along the processes of the FRC network in the T-cell zone, exposing them to Ag-bearing DCs strategically positioned along these migration routes.18
When B cells enter the follicles where FRCs are very sparse, they encounter and migrate in contact with the dense follicular DC (FDC) network.18 In addition to this role in B-cell migration, FDCs provide several other crucial functions for B cells. First, FDCs produce soluble factors such as the B-cell activator factor of tumor necrosis factor (TNF) family (BAFF) and CXC chemokine ligand 13, a B-cell survival factor and a follicle-homing chemokine, respectively.19 Second, FDCs capture and present immune complexes (ICs) to B cells, allowing the formation and maturation of the germinal center, a structure in which B cells proliferate, undergo somatic hypermutation, and carry out class switching.19 How do ICs and particulate Ags reach the FDC network to be presented to follicular B cells? A subset of macrophages present in the SCS (SCM) is involved in this transport. SCMs are akin to the marginal zone metallophilic macrophages present in the spleen.2,4 Whereas marginal zone metallophilic macrophages filter the blood content flowing in the spleen, SCMs filter the lymphatic content conveyed to LNs. Because of their long dendrites, SCMs are ideally situated to transfer particulate Ags to the underlying follicles without migrating. Using imaging techniques, 3 groups have shown that SCMs can transfer such Ags and ICs to B cells, providing a third way for B cells to encounter their ligands.20-22
In this study, we attempt to relate the data on the distinct conduit architecture of the T and B zones with these various reports on the different modes of Ag delivery to B cells within the follicle. We present evidence that during ontogeny, B-cell follicles develop at the periphery of the T-cell zone, in regions in which the conduit system is already formed, functional, and dense. The continued expansion of the incipient follicles is accompanied by a progressive remodeling of the conduit network within the domain of the growing follicle, a process that ultimately leads to a sparse network that nonetheless maintains its connectivity. Over the course of this remodeling, FRCs disappear around the remaining follicular conduits still connected to the SCS and, at least in the deeper regions of the follicle, are replaced by the FDC network newly generated by the accumulation of B cells. The strategic positioning of FDCs around follicular conduits correlates with their capacity to capture soluble Ags draining from subcutaneous sites even in the absence of Ag-specific Abs. These data suggest that the B-cell follicles “hijack” the function of the preexisting conduit system produced by the FRCs, allowing their own stromal cell population, the FDCs, access to lymphatic content that can be transferred to B cells moving in contact with their surface. Our findings help explain the distinct organization of the conduit networks in the T and B zones of mature LNs and suggest how each is specialized to provide Ags to different cohorts of naive lymphocytes migrating in the respective regions.
Methods
Mice
C57BL/6 were purchased from Janvier, and C57BL/6 ubiquitin–green fluorescent protein (GFP) mice (UBI-GFP/BL6, strain 4353), recombination-activating gene-1–deficient mice (strain 2216), hen egg lysozyme (HEL)–specific B-cell receptor transgenic mice (MD4, strain 2595), and B cell–deficient mice (μMt, strain 2288) were purchased from The Jackson Laboratory and maintained in the National Institutes of Health and Institut de Pharmacologie Moléculaire et Cellulaire animal facilities. For the generation of chimeras, recombination-activating gene-1–deficient C57BL/6 ubiquitin-GFP mice were γ-irradiated with a single dose of 950 rad (or twice with 500 rad) from a cesium source and were reconstituted with 2 × 106 C57BL/6 bone marrow cells. At 8 weeks after reconstitution, mice were tested for chimerism. Chimeras were used for subsequent experiments only if analysis of blood leukocytes showed the presence of less than 2% of CD3+ T cells of host origin. Mice were injected in the ears with 10 μL of Ags or fluorescent tracer. All procedures performed on animals in this study have been approved by the Animal Care and Use Committee, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and/or the Inserm, Université de Nice-Sophia Antipolis.
Adoptive transfers
B cells were purified from the LNs/spleens of wild-type (WT) mice using a pan B-cell isolation kit (Miltenyi Biotec) and in the indicated situations stained with 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen) at 37°C for 15 minutes. The indicated numbers of cells were transferred into host mice by intravenous injection.
Antibodies
ERTR-7 antibody specific for an unknown FRC-secreted molecule, Lyve-1 antibody specific for lymphatic vessels, and polyclonal antibodies specific for desmin were purchased from Acris Antibodies. FDC-M2 antibody specific for FDCs was purchased from Immunokontact. MOMA-1 antibody specific for metallophilic macrophages was purchased from Cedarlane Laboratories. The antibody specific for collagen IV was purchased from Abcam. RA3-6B2 antibody specific for B220 and 17A2 specific for the CD3 complex were purchased from BD Biosciences. The antibody specific for HEL and ovalbumin (OVA) were purchased from Rockland Immunochemicals and Sigma-Aldrich, respectively. Primary antibodies were visualized by direct coupling to allophycocyanin, Alexa Fluor-488, Alexa Fluor-568, and Alexa Fluor-647. When necessary, unconjugated primary antibodies were used and revealed by either Alexa Fluor-488–, Alexa Fluor-568–, or Alexa Fluor-647–coupled secondary antibodies or biotinylated antibodies, followed by fluorescent-conjugated streptavidins or Tyramide Signal Amplification Kits from Invitrogen.
In vivo depletion of SCS macrophages
WT mice were injected with 30 μL of chlodronate- or phosphate-buffered saline (PBS)–loaded liposomes (kindly provided by Clodronate Liposomes) in the hind footpads. SCS macrophage depletion of the popliteal LNs was complete by 6 days postinjection, and for this reason, all experiments performed in the absence of SCS macrophages were conducted at this time point.
Immunostaining
LNs were incubated in 15 mL of 0.05M phosphate buffer containing 0.1M l-lysine, pH 7.4, 2 mg/mL NaIO4, and 10 mg/mL paraformaldehyde. Twelve hours later, LNs were washed in phosphate buffer and dehydrated in 30% sucrose in phosphate buffer. Tissues were snap frozen in Tissue-Tek (Sakura Finetek). Frozen sections (10-40 μm) were cut and then stained with the indicated antibodies, as previously described.16 For anti-HEL staining, LNs were snap frozen in OCT, cut on a cryostat, and fixed for 20 seconds in acetone before staining. Immunofluorescence confocal microscopy was performed using a Leica TCS SP5 confocal microscope. Separate images were collected for each fluorochrome and overlaid to obtain a multicolor image. Final image processing was performed using ImageJ software (National Institutes of Health) and Adobe Photoshop.
Quantification of the reticular fiber network in B- and T-cell areas
Immunofluorescence images were segmented into B- and T-cell areas (without their respective blood vessels that are not part of the reticular fiber network) using ImageJ software (National Institutes of Health), and the number of pixels specific for ERTR-7 was measured and expressed as the percentage of total pixels in each area.
Results
B-cell follicle expansion is associated with remodeling of the conduit system
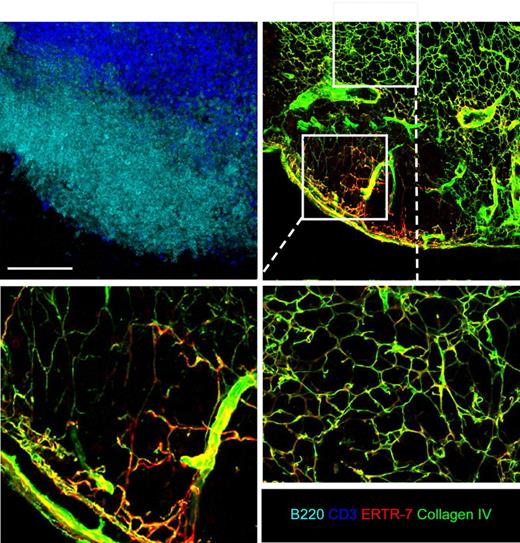
Consistent with its major roles in guiding naive T-cell movements, T-DC interactions, and Ag delivery to DCs,10,18 the conduit network is dense in the paracortex of LN, but only sparsely present in the B-cell follicles.6,18 In the interfollicular region separating the FRC-rich T-cell zone and the FRC-poor B follicles, Katakai et al described a special region called the cortical ridge (CR). Structurally, the CR is a portion of the T/B interface area in which the conduit system is denser that the one present in the rest of the T-cell zone (supplemental Figure 1, available on the Blood website; see the Suppplemental Materials link at the top of the online article).6 As recently observed by Roozendaal et al, the conduit system is present under the SCS and invades the follicle, where it is quite sparse compared with the paracortex.13 This observation raises the issue of what leads to this regionally distinct organization of the conduit network during LN development. As a first approach to addressing this question, 40-μm tissue sections of LNs from WT animals were stained with antibodies specific for B cells (B220) and reticular fibers (ERTR-7 and collagen IV) and analyzed by confocal microscopy (Figure 1). A careful analysis of these thick sections revealed that despite their paucity, the follicular conduits are well interconnected in the B-cell follicles. However, whereas the conduit system in the T-cell zone and just underneath the capsule forms a highly branched, densely connected network, the sparse follicular network is composed primarily of long and poorly branched fibers connecting the SCS to the CR.
The conduit system of B-cell follicles is sparse and poorly branched. Peripheral LNs (except mesenteric) were collected from WT mice and cut in 40-μm-thick sections. LN sections were stained for B220 (light blue), CD3 (deep blue), ERTR-7 (red), and collagen IV (green) expression to reveal the complex structure of the conduit network present in the T- and B-cell zone when analyzed by confocal microscopy. Representative LN sections show the differences of the conduit network in the B- and the T-cell zones, as highlighted in the insets showing enlarged views of the conduits in the 2 areas. Data were acquired using a Leica Sp5 microscope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
The conduit system of B-cell follicles is sparse and poorly branched. Peripheral LNs (except mesenteric) were collected from WT mice and cut in 40-μm-thick sections. LN sections were stained for B220 (light blue), CD3 (deep blue), ERTR-7 (red), and collagen IV (green) expression to reveal the complex structure of the conduit network present in the T- and B-cell zone when analyzed by confocal microscopy. Representative LN sections show the differences of the conduit network in the B- and the T-cell zones, as highlighted in the insets showing enlarged views of the conduits in the 2 areas. Data were acquired using a Leica Sp5 microscope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
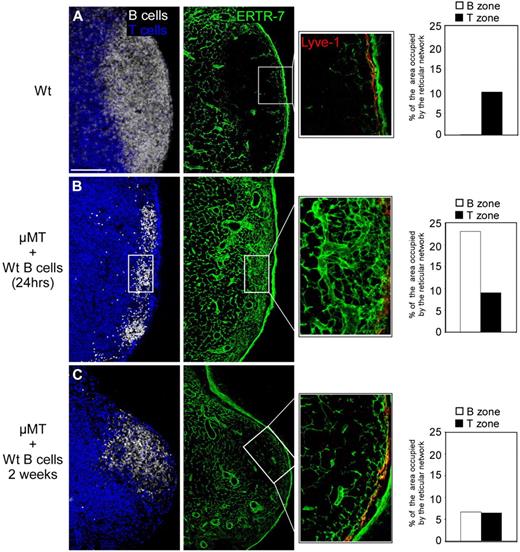
In seeking to explain these observations, we hypothesized that developing B follicles might expand their territory without producing the new FRCs and collagen fibers needed to maintain a dense and connected conduit network. Alternatively, the enlarging follicles may push the conduit network out of this zone of growth. Because the LN capsule is thick and rigid, we reasoned that such a repulsion of the network would compress it at the T/B interface, leading to the creation of the conduit-rich CR described by Katakai et al.6 To examine how follicle growth is related to the structure of the conduit network, we first examined the status of the network in μMT mice that lack mature B cells and FDCs.23,24 We adoptively transferred 2 × 107 CMFDA-labeled WT B cells into μMT mice and harvested the LNs 1 day and 2 weeks later, at a time when the transferred B cells start to form follicles. LNs were then cut; stained with antibodies specific for B cells (B220), reticular fibers (ERTR-7), and lymphatic vessels (Lyve-1); and analyzed by confocal microscopy. After 1 day, the transferred B cells gathered in small focal structures under the SCS, allowing us to identify the location in which B-cell follicles will later develop. Interestingly, the conduit system was very dense in these regions compared with a WT animal of the same age (Figure 2, A vs B). Two weeks after the transfer of WT B cells into μMT mice, the developing follicles could easily be identified. A close examination of these follicles revealed that the conduit system had become sparse in the volume occupied by the enlarging B-cell follicle (Figure 2C). These results reveal that B-cell follicle expansion is accompanied by a dramatic change in the local architecture of the pre-existing conduit network, giving rise to thin and poorly branched fibers compared with those present in the T zone within which these follicles emerge.
The conduit network is remodeled within developing B follicles. B cell–deficient (μMT) mice were injected with 2 × 107 CMFDA-labeled WT B cells. Peripheral LNs (except mesenteric) were collected from WT mice (A) and μMT mice 1 day (B) and 2 weeks (C) after transfer. Sections were stained for B220 (white), CD3 (blue), ERTR-7 (green), and Lyve-1 (red) to reveal the status of the conduit network present in the T- and B-cell zone when analyzed by confocal microscopy. Histograms indicate the percentage of B-cell areas and T-cell areas occupied by the reticular fibers in each condition. Data were acquired using a Leica Sp5 microscope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
The conduit network is remodeled within developing B follicles. B cell–deficient (μMT) mice were injected with 2 × 107 CMFDA-labeled WT B cells. Peripheral LNs (except mesenteric) were collected from WT mice (A) and μMT mice 1 day (B) and 2 weeks (C) after transfer. Sections were stained for B220 (white), CD3 (blue), ERTR-7 (green), and Lyve-1 (red) to reveal the status of the conduit network present in the T- and B-cell zone when analyzed by confocal microscopy. Histograms indicate the percentage of B-cell areas and T-cell areas occupied by the reticular fibers in each condition. Data were acquired using a Leica Sp5 microscope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
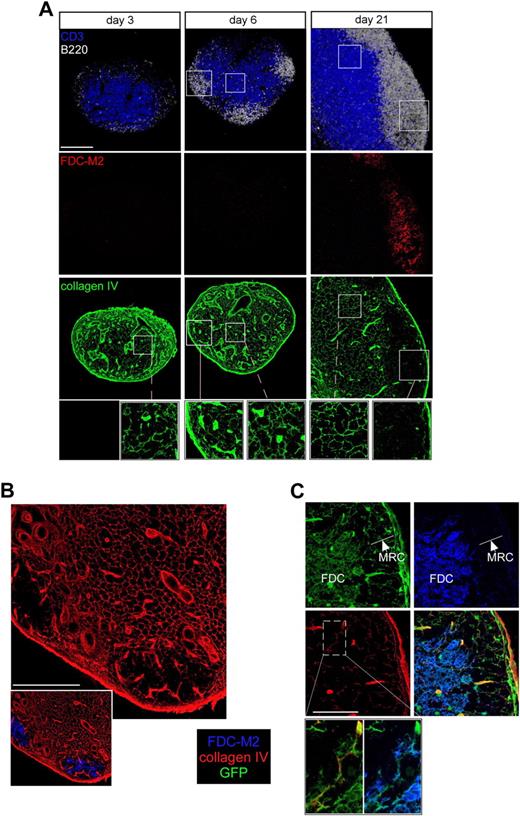
To determine whether the same changes in conduit density characterized the physiologic process of LN development, we relied on observations showing that immature immunoglobulin M− or immunoglobulin G− B cells are present at day 2 after birth in the rat, but that B-cell follicle formation occurs only at day 18, much later than the establishment of the T-cell zone, which is already present at birth.25 Therefore, we collected LNs from day 3 to day 21 after birth and fixed, sectioned, and stained these tissues for collagen IV, FDC-M2 (a FDC-specific marker), B220, and CD3 expression. The stained sections were then examined by confocal microscopy. On postnatal day 3, LNs already possessed a T-cell zone and a well-developed system of conduits, but very few B cells (Figure 3A). The first follicle-like structures appeared on day 6 at the periphery of the central T-cell zone, but did not possess developed FDC networks based on FDC-M2 expression. At this stage of postnatal development, the regions in which B cells gathered in small follicles possessed a dense conduit network (Figure 3A insets). However, by day 21 after birth, numerous FDC-rich follicles had developed that now had only a sparse conduit network. These latter observations of normal ontogeny are congruent with the preceding cell transfer studies and support the notion that the regions of LNs in which B follicles develop begin with a rich conduit network that is lost as the follicle enlarges during development.
Follicle development is associated with conduit remodeling in neonates and allows FDCs to wrap around conduits. (A) WT mice were killed at day 3, 6, or 21 after birth. Axillary and inguinal LNs were harvested, sectioned, and stained for B220 (white), CD3 (blue), collagen IV (green), and FDC-M2 (red) to analyze by confocal microscopy the status of the conduit system in the developing follicles over time. Insets show enlargements of B- and T-cell areas. (B-C) LNs from adult irradiated ubiquitin-GFP mice reconstituted with WT bone marrow were sectioned and stained for collagen IV (red) and FDC-M2 (blue) expression to distinguish by confocal microscopy the very thin processes of the radioresistant GFP-positive (green)–expressing cells present in the follicles. Insets in panel C show an enlargement of a conduit and its enwrapping FDCs in the follicle. Data were acquired using a Leica Sp5 microscope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
Follicle development is associated with conduit remodeling in neonates and allows FDCs to wrap around conduits. (A) WT mice were killed at day 3, 6, or 21 after birth. Axillary and inguinal LNs were harvested, sectioned, and stained for B220 (white), CD3 (blue), collagen IV (green), and FDC-M2 (red) to analyze by confocal microscopy the status of the conduit system in the developing follicles over time. Insets show enlargements of B- and T-cell areas. (B-C) LNs from adult irradiated ubiquitin-GFP mice reconstituted with WT bone marrow were sectioned and stained for collagen IV (red) and FDC-M2 (blue) expression to distinguish by confocal microscopy the very thin processes of the radioresistant GFP-positive (green)–expressing cells present in the follicles. Insets in panel C show an enlargement of a conduit and its enwrapping FDCs in the follicle. Data were acquired using a Leica Sp5 microscope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
FDCs wrap around the remaining conduit system in B-cell follicles
In the T-cell zone, the conduit system is ensheathed by FRCs, which helps ensure an appropriate seal around the lymph-transporting conduit system.10 Because follicle development alters the organization of the conduit system and simultaneously induces the formation of the FDC network, we examined whether FRCs still wrapped around the remaining conduits or whether FDCs replaced these other stromal cells. To observe the thin processes of both types of stromal populations within the follicles, we generated chimeric mice using WT bone marrow cells to reconstitute irradiated ubiquitin promoter-GFP transgenic animals in which the fluorescent protein is expressed by all nucleated cells.26 Animals were allowed to reconstitute for a minimum of 8 weeks to ensure nearly complete replacement of hematopoietic-derived cells with nonfluorescent populations. As previously described, FDC and FRC networks expressed GFP in their respective areas.18 LN sections from such animals were stained for collagen IV, FDC-M2, and B220 expression and analyzed by confocal microscopy (Figure 3B-C). The results showed that the FDC network predominantly colonized the central region of the follicle where the conduit system was very sparse (Figure 3B). A closer examination of the region located just underneath the SCS showed that GFP+ stromal cells present in this region did not express FDC-M2, wrapped around the conduits, and were thus likely to be the marginal reticular cells (MRCs) recently described by Katakai et al.27 In contrast, deeper in the follicles, the GFP+ stromal cells that ensheathed the conduits expressed FDC-M2, indicating that in the follicles, the residual conduits were no longer surrounded by FRCs or MRCs, but rather by FDCs (Figure 3C).
Soluble Ags can be transported in the follicles by the residual conduit system and captured by the FDC network
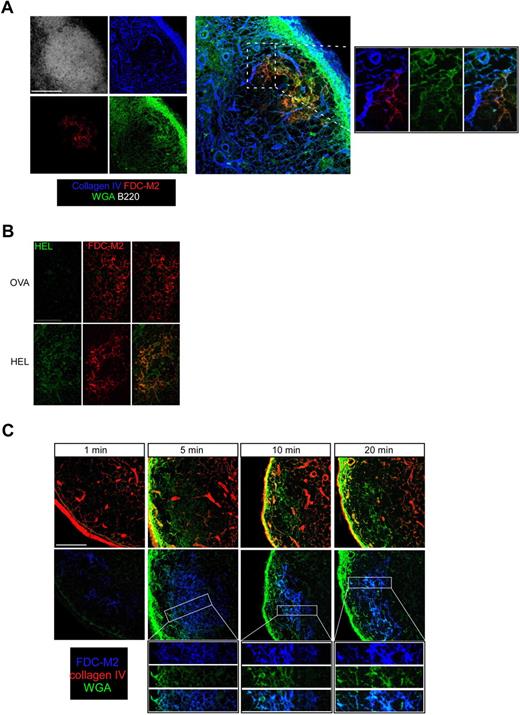
The conduit system transports the lymph from the SCS to the medulla through the T-cell zone, allowing the numerous DCs settled on the FRC network to sample its content.10 In the follicles, the conduits emerging from the SCS are sparse and are no longer wrapped by FRCs, but rather by FDCs. This arrangement could allow FDCs to gain access to the lymphatic content. Pape et al reported that visualizing soluble Ags in the absence of cognate antibody that would produce ICs that bind to FDC complement receptors is technically challenging using conventional immunostaining techniques that flush the Ags away.14 Therefore, we decided to use fluorescent wheat germ agglutinin (WGA) as an Ag analog because this lectin binds to cells and is retained by them.9 In addition, WGA is small (38 kDa) and has been shown to be efficiently transported by the conduits.9 WT mice were subcutaneously injected with labeled WGA, and 30 minutes later draining LNs were fixed; sectioned; stained for collagen IV, FDC-M2, and B220 expression; and analyzed by confocal microscopy. As shown in Figure 4A, WGA and collagen IV signals overlapped in the follicles, confirming that WGA was efficiently transported by the follicular conduits, as recently described.13 In addition, in the follicle, WGA fluorescence was also detected in a second reticular network positive for FDC-M2 expression, indicating that FDCs rapidly captured a soluble protein injected subcutaneously.
FDCs rapidly capture a soluble Ag injected subcutaneously. (A) WT mice were injected with 10 μL (50 μg) of Alexa 488–conjugated WGA (green) in the ears. Thirty minutes later, ear-draining LNs were collected; sectioned; stained for B220 (white), FDC-M2 (red), and collagen IV (blue) expression; and analyzed by confocal microscopy. Insets show the enlargement of a region that contains FDCs and conduits. (B) WT mice were injected with 10 μL (20 μg) of OVA or HEL in the ears. Ear-draining LNs were harvested 30 minutes later, sectioned, stained for HEL (green) and FDC-M2 (red) expression, and analyzed by confocal microscopy. (C) WT mice were injected with 10 μL (50 μg) of Alexa 488–conjugated WGA (green) in the ears. One, 5, 10, and 20 minutes later, ear-draining LNs were collected, sectioned, and stained for FDC-M2 (blue) and collagen IV (red) expression to examine the WGA diffusion over time when analyzed by confocal microscopy. Data were acquired with a Leica Sp5 microcope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
FDCs rapidly capture a soluble Ag injected subcutaneously. (A) WT mice were injected with 10 μL (50 μg) of Alexa 488–conjugated WGA (green) in the ears. Thirty minutes later, ear-draining LNs were collected; sectioned; stained for B220 (white), FDC-M2 (red), and collagen IV (blue) expression; and analyzed by confocal microscopy. Insets show the enlargement of a region that contains FDCs and conduits. (B) WT mice were injected with 10 μL (20 μg) of OVA or HEL in the ears. Ear-draining LNs were harvested 30 minutes later, sectioned, stained for HEL (green) and FDC-M2 (red) expression, and analyzed by confocal microscopy. (C) WT mice were injected with 10 μL (50 μg) of Alexa 488–conjugated WGA (green) in the ears. One, 5, 10, and 20 minutes later, ear-draining LNs were collected, sectioned, and stained for FDC-M2 (blue) and collagen IV (red) expression to examine the WGA diffusion over time when analyzed by confocal microscopy. Data were acquired with a Leica Sp5 microcope (×20 and ×63 objectives) and are representative of 3 different experiments. Scale bar: 100 μm.
We next investigated whether the capture of WGA by FDCs was a specific feature of the lectin or whether other Ags such as HEL or OVA could also be rapidly transported to and bound by FDCs. WT mice were injected intradermally in the ears with 20 μg of OVA or HEL and their ear-draining LNs dissected 30 minutes later. LNs were sectioned and stained for FDC-M2 and HEL expression. Data show that HEL signal was detected in HEL-, but not OVA-injected mice and overlapped with the FDC-M2 signal in the follicles, indicating that HEL is efficiently transported in the FDC network (Figure 4B). When stained with an anti-OVA Ab, a signal overlapping with the FDC-M2 signal was detected in OVA- but not in HEL-injected mice (data not shown), indicating that it is not just the unusual cationic properties of HEL or the lectin properties of WGA that account for protein capture by FDCs.
We then performed a time course experiment to examine the kinetic of the WGA capture by FDCs. WGA binding was evident as soon as 5 minutes after WGA injection (Figure 4C). Interestingly, the FDC network became labeled in a centripetal fashion over time, with the external FDC processes close to the SCS becoming labeled 5 minutes after the injection, whereas the labeling of the central FDCs required 10 to 20 minutes to be as intense, consistent with progressive transport of the soluble tracer to the center of the follicle. To examine the possibility that natural occurring Abs may be responsible for the capture of soluble Ags by FDCs, we injected WGA or OVA into transgenic MD4 mice in which all B cells express a B-cell receptor specific of the HEL. Removal of the draining LNs 30 minutes later and analysis of sections by confocal microscopy indicated that FDCs from OVA- or WGA-injected MD4 mice captured the injected Ags (data not shown), suggesting that FDCs can capture soluble Ags and not just ICs.
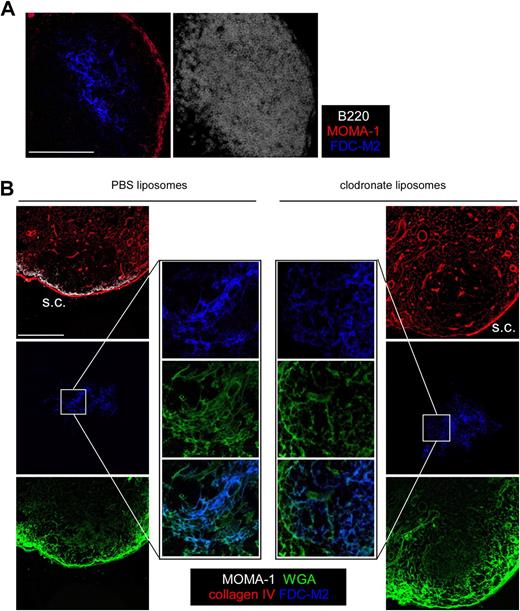
MOMA-1–expressing SCMs have been shown to transfer particulate Ags and ICs to B cells.20-22 As a consequence, it was possible that SCMs were involved in the deposition of the fluorescent WGA on the FDCs. MOMA-1 SCMs reside in the SCS, but can extend dendrites into the follicle via the SCS floor, although these dendrites do not seem to interact directly with FDCs (Figure 5A). To examine whether the FDC WGA deposition we observed was due to the activity of the SCMs, we used the ability of chlodronate-loaded liposomes to deplete the SCMs of the draining LN when injected subcutaneously.28 PBS or chlodronate liposomes were injected in the ears of WT mice. Six days later, when SCM depletion of the draining LN is complete, mice were injected in the ears with fluorescent WGA. LNs were harvested 30 minutes later; sectioned; stained for MOMA-1, FDC-M2, and collagen IV expression; and analyzed by confocal microscopy. As shown in Figure 5B, whereas chlodronate (but not PBS) liposome injection efficiently depleted the LN rim of SCM, it did not prevent the WGA capture by FDCs, indicating that WGA transport from SCM to FDCs is not the main process responsible for soluble Ag deposition on FDCs. These data indicate that not only Ags contained in ICs, as previously reported,19 but also noncomplexed Ags, can be captured by FDCs that surround follicular conduits, and that such capture is independent of the SCS macrophages involved in presentation of particulate Ags to B cells.
SCS macrophages are not responsible for Ag transport to FDCs. (A) LN sections from WT mice were stained for B220 (white), FDC-M2 (blue), and MOMA-1 (red), then analyzed by confocal microscopy to highlight the SCS macrophages and their dendritic protrusions that penetrate the follicles. (B) WT mice were injected with 30 μL of PBS- or chlodornate-loaded liposomes in the hind footpads. Six days later, when SCS macrophage depletion was complete, 10 μL (50 μg) of Alexa 647–conjugated WGA was injected in the footpads of the treated animals. Popliteal LNs were harvested 30 minutes later; sectioned; stained for MOMA-1 (white), collagen IV (red), and FDC-M2 (blue); and then analyzed by confocal microscopy. Data were acquired with a Leica Sp5 microcope (×20 and ×63 objectives) and are representative of 2 different experiments. Scale bar: 100 μm.
SCS macrophages are not responsible for Ag transport to FDCs. (A) LN sections from WT mice were stained for B220 (white), FDC-M2 (blue), and MOMA-1 (red), then analyzed by confocal microscopy to highlight the SCS macrophages and their dendritic protrusions that penetrate the follicles. (B) WT mice were injected with 30 μL of PBS- or chlodornate-loaded liposomes in the hind footpads. Six days later, when SCS macrophage depletion was complete, 10 μL (50 μg) of Alexa 647–conjugated WGA was injected in the footpads of the treated animals. Popliteal LNs were harvested 30 minutes later; sectioned; stained for MOMA-1 (white), collagen IV (red), and FDC-M2 (blue); and then analyzed by confocal microscopy. Data were acquired with a Leica Sp5 microcope (×20 and ×63 objectives) and are representative of 2 different experiments. Scale bar: 100 μm.
Discussion
In adult mice, there is a marked difference in the extent and organization of the stromal cell-dependent conduit network in B- and T-cell areas of LNs. The conduit system is rich and highly branched within the paracortical T zone, but sparse and poorly branched in the B follicles. In this study, we have investigated the origin and possible functional consequences of these differences, as well as the respective positioning and roles of FRC versus FDC in the 2 lymphoid subcompartments. Our data reveal that the stromal cell network and conduit system of LNs change dramatically during postnatal lymphorganogenesis. During LN colonization with mature lymphocytes, B cells accumulate and generate follicles long after the paracortex has been well occupied by T cells. Thus, at early times (the first week after birth) when the LN is filled mostly by T cells, the FRC-associated conduit network is extensive and highly branched in the parenchyma of the node. This dense, connected organization is maintained as the LN grows with the entry of additional T and B cells. In contrast, as B cells enter and begin to form primary follicles, the conduit network in the growing follicular region undergoes progressive remodeling. This results in both a reduction in the density and branching pattern of the conduits that remain within the follicle borders. The presence of this sparse, but connected network accounts for lymph transport into the region of the follicle proximal to the SCS, as has recently been observed by Roozendaal et al,13 and presumably also explains the observations of Pape et al on soluble Ag entry after subcutaneous injection.14
At the same time, follicle formation induces FDC maturation, leading to the creation of a dense and intricate 3-dimensional FDC network. FRCs no longer wrap around the conduits that remain in the B follicle, as they do around those in the T-cell zone. Instead, FDCs replace the FRCs in this strategic location. In agreement with Roozendaal et al,13 we found that after a subcutaneous injection of a fluorescent soluble protein, the tracer is transported within minutes into the follicles via the conduit system. We observed that such transport leads to the capture of the tracer by FDCs in the absence of specific antibody. These latter results suggest an additional mechanism to Ag delivery by FDCs to specific B cells, namely the direct acquisition by contact or local release of free Ag, not just the capture of immune complexes from the FDC surface.
How small free soluble Ags are transported to the follicle and delivered to B cells has been the subject of controversy for many years. The observation of small pores in the floor of the SCS has led to the assumption that soluble Ags are capable of traversing these openings and diffusing throughout the follicle. However, the very presence of these pores is still controversial and questions whether and to what extent passive diffusion across the SCS floor occurs.2-5 In addition, 2 groups have observed B-cell activation hundreds of microns below the SCS within minutes after a subcutaneous injection of Ags.13,14 B-cell follicles are densely populated, questioning how free diffusion in the parenchyma of the LN could bring soluble Ags to the B cells located in the deep follicle so quickly. Roozendaal et al15 recently demonstrated that small conduits connected to the SCS transport small soluble Ags directly into the follicles. As the authors observed that the Ag-specific B cells located near the conduits acquired the Ags first, they concluded that the Ag is transported via the conduits and either made available through small openings in the conduit sheath that B cells could probe or released in the interstitium where B cells would gain access, explaining how B cells can be activated within minutes of a subcutaneous injection of soluble Ag.13 Our data do not disagree with this hypothesis, but rather suggest that B cells may also obtain ligands directly from Ag-decorated FDCs.
During an infection, it is likely that limiting amounts of Ags will be provided to the LN. Thus, if Ag is leaking from the follicular conduits or flowing from the SCS, it would be present in the interstitium of the follicle in small quantities and for a short period of time before being rapidly washed away by the continuous flow of afferent lymph. In such a situation, a rare Ag-specific B cell patrolling another lymphoid organ may be recruited in the draining LN too late to sense its Ags in a soluble form. Having a source of Ags captured by FDCs even in the absence of preexisting (natural) Ab would ensure a longer Ag availability for Bcells, allowing late-arriving Ag-specific B cells to find their ligand even if it is no longer conveyed by the lymph. Another advantage of immobilizing Ags on FDCs relates to the fact that B cells migrate in intimate contact with the FDC network, thus maximizing the likelihood of a B cell encountering any Ags deposited on these stromal cells. Whereas previous work has emphasized the importance of Ags in the form of ICs on the FDC surface, in this study we suggest the possibility of direct Ag presentation by these nonhematopoietic elements.
In the T-cell zone, Ag and low-molecular-weight immune mediators are transported via the interconnected branches of the conduit system ensheathed by FRCs, which limits free access of these materials to the surrounding parenchyma. In the follicles, the conduits also appear to form a closed system. We raise the hypothesis that FDCs may be an important regulator of free diffusion of Ag and inflammatory signals by helping to maintain the protected nature of the conduit system within the follicle. This process may rely on the FDC ability to actively transport molecules from the luminal side of the conduit or on their capacity to capture Ags leaking out of the gaps reported by Roozendaal et al.13 Further studies will be required to test these hypotheses.
Apart from the possible role for FDC presentation of Ags highlighted above, a major aspect of the present study is the new evidence for dramatic remodeling of the conduit and stromal cell network in LNs during B-follicle development. Interestingly, the remodeling of the conduit system, the disappearance of the FRC network, and the appearance of the FDC network are synchronous events during follicle enlargement. It is not yet clear whether B cells per se and/or the FDC development they induce are responsible for the remodeling phenomenon, nor which soluble and/or surface molecules might be involved. FDC network survival depends on continuous signaling by several members of the TNF family, such as lymphotoxin or lymphotoxin β provided by B cells.19 As a consequence, FDCs are present neither in B cell–deficient mice nor in mice deficient for these TNF family members.24,29,30 Alternatively, FDCs are responsible for the proper formation of B-cell follicles, and their absence implies a severe B-cell deficiency.24 Therefore, to our knowledge, it is impossible to generate mice in which one subset is present in the absence of the other, preventing the identification of the subset that is involved in the disruption of the conduit/FRC system during LN colonization.
Several nonmutually exclusive mechanisms may ultimately lead to the progressive disappearance of the follicular conduit system and its associated FRCs. First, FRC apoptosis may be induced upon B-follicle development. Alternatively, progressive FRC disappearance from the developing follicles may not result from cell death, but rather from an incapacity of FRC or their precursors to proliferate in these structures. Neonatal LN rapidly enlarges upon the postnatal influx of lymphocytes. As the FRC network maintains its connectivity and density in the developing T-cell zone, new FRCs must be rapidly generated in or migrate into that zone during the first days after birth. Therefore, if FRC proliferation or recruitment signals are not provided within developing B-follicular zones, it would not be possible to retain the density of the network characteristic of the T zone. Because lymphoid tissue-inducer cells are important regulators of the generation, but not maintenance of FRC,31,32 it is possible that their inhibition, death, or repulsion in the developing follicle during the first days of life would result in the apparent shrinkage of the FRC network in adult LNs.
FRCs and FDCs are akin in several ways, sharing numerous physical and chemical similarities. Both subsets belong to the LN mesenchymal stroma and form networks of interconnected cells with a dendritic shape. In addition, they produce and present chemokines that regulate similar functions in the lymphocyte subsets with which they intermingle. CCL21/CCL19 secretion by FRCs is important for T-cell entry into the T-cell zone, whereas CXC chemokine ligand 13 secretion by FDCs regulates B-cell entry in follicles.33,34 Both subsets secrete survival factors for their respective lymphocytes, with FDCs secreting the B-cell survival factor B-cell activator factor of TNF family35,36 and FRCs producing the T-cell survival factor interleukin-7.37 Finally, we have recently shown that FRC and FDC networks support T/B- and B-cell migration in LNs.18 Based on these features, the fact that the FDC network develops when the FRC network disappears in the B-cell follicle, and our observation that FDCs ensheath the remaining conduits like FRCs in the T-cell zone, an intriguing possibility is that a subset of FRCs (or MRCs) is reprogrammed into FDCs during follicle development. However, it is important to notice that 24 hours after their adoptive transfer into μMT mice, B cells do not disperse in the whole T-cell zone, but rather aggregate in clusters at the periphery of the T-cell zone. This suggests that an unknown cell type present in the center of these clusters (FDC precursor, subset of FRCs or MRCs, etc) attracts B cells and is important for follicle development, or that there is a local intersection of chemokine gradients especially suitable to localization of B cells migrating within the LN parenchyma after HEV egress. Finally, the conduit and FRC disappearance may simply result from the physical pressure imposed by the numerous B cells accumulating in the nascent follicle during ontogeny, explaining the existence of the cortical ridge and its dense network.6
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Julie Cazareth and Frédéric Brau for their help; Valérie Verhasselt for providing the newborn mice; and Jackson G. Egen, Grégoire Lauvau, and Nicolas Glaichenhaus for their extremely helpful advice on the manuscript.
This work was supported by grants from Inserm, Centre National de la Recherche Scientifique, Agence Nationale de la Recherche, and funds from the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
National Institutes of Health
Authorship
Contribution: M.B. and R.N.G. designed the experiments and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Bajénoff, Centre d'Immunologie de Marseille Luminy, Parc Scientifique et Technologique de Marseille Luminy, Case 906, F13288 Marseille, France; e-mail: bajenoff@ciml.univ-mrs.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal