Abstract
Immune-related deficiencies are well-known complications of chronic lymphocytic leukemia (CLL). Although recent data indicate that almost all CLL patients are preceded by a monoclonal B-cell lymphocytosis precursor state, patterns of immune defects preceding CLL diagnosis are unclear. We identified 109 persons who developed CLL from the prospective and nationwide Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial with 77 469 participants, with serially collected prediagnostic serum samples. We assayed monoclonal (M)–proteins, κ/λ free light chains (FLCs) in prediagnostic obtained up to 9.8 years before CLL diagnosis. The prevalence of an abnormal FLC ratio, M-protein, and hypogamma-globulinemia before CLL diagnosis was 38% (95% confidence interval, 29%-47%), 13% (7%-21%), and 3% (1%-8%), respectively. M-proteins and abnormal FLC ratios were detected up to 9.8 years before CLL diagnosis in a total of 48 persons (44%). Hypogammaglobulinemia was not present until 3 years before the diagnosis of CLL. Among 37 patients with information on tumor cell immunophenotype, an association between immunophenotype and involved FLC (P = .024, Fisher exact test) was observed. Among 61 persons with a normal FLC ratio and without an M-protein, 17 had elevated κ and/or λ FLC levels, indicating polyclonal B-cell activation in 17 of 109 (16%) patients. These findings support a role for chronic immune stimulation in CLL genesis.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by an accumulation of mature B lymphocytes.1 It accounts for approximately 30% of all leukemia and is the most common form of leukemia among adults in Western countries.2 In a recent study based on 77 469 healthy adults who were enrolled in the nationwide, population-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial,3 we identified 45 subjects with peripheral whole-blood collection and a diagnosis of CLL up to 6.4 years after blood collection draws. Using 6-color flow cytometry and immunoglobulin heavy-chain gene rearrangement (IGHV) by reverse-transcriptase polymerase chain reaction assay, we found evidence of prediagnostic monoclonality among B cells (by either of the 2 methods) in 44 patients (98%; 95% confidence interval [CI], 88%-100%), up to 6.4 years before the initial diagnosis. In 41 patients (91%; 95% CI, 79%-98%), the clone was confirmed by both methods.3 The distribution of mutated clones, compared with unmutated clones, was very similar regardless of the time at which the blood samples was obtained and the subsequent CLL diagnosis. In addition, although based on small numbers, among the 8 unmutated prediagnostic clones, 3 were present more than 3 years before the CLL diagnosis, with 2 being detectable 5 years before. Thus, this study suggests that virtually all cases of CLL (both with mutated and unmutated IGHV genes) are preceded by monoclonal B-cell lymphocytosis.
Immune-related phenomena are well-known complications of CLL; however, whether immune disruption actually precedes CLL diagnosis is not known. In addition, based on small numbers, a few hospital-based studies have described evidence of monoclonal (M)–protein and hypogammaglobulinemia around the time of CLL diagnosis.4,5 Interestingly, a prior study based on 111 CLL patients found that those with an M-protein at diagnosis had a poorer survival than those without (63 months vs 103 months; P < .04).4 Furthermore, a recent study including 181 CLL patients found an abnormal free light chain (FLC) ratio to be independently associated with a poor prognosis along with 3 other previously established markers (Zap-70 expression, elevated β-2-microglobulin levels, and unmutated IGHV mutational status).6 These observations suggest that detectable serum protein abnormalities in CLL may reflect biologic heterogeneity, presenting by differential prognostic profiles.
To improve our understanding of prediagnostic immune defects in CLL, we took advantage of the large nationwide US PLCO Cancer Screening Trial.7 To our knowledge, this is the first prospective population-based study to evaluate patterns of serum protein abnormalities in the pathway to CLL. Among more than 77 000 persons who were cancer-free at study start, we identified all persons who subsequently were diagnosed with CLL and who had available stored serum samples. Using available stored blood samples obtained up to 9.8 years before and near the CLL diagnosis, we were able to evaluate the presence and temporal patterns of κ and λ FLCs, M-proteins, and hypogammaglobulinemia present before CLL.
Methods
Study population and CLL patients
The study population of the PLCO Cancer Screening Trial was described previously.7 Briefly, more than 150 000 persons 55 to 74 years of age from 10 study centers across the United States were randomized between 1992 and 2001 to undergo a specific cancer screening regimen (screening arm) or receive routine medical care (control arm) to evaluate the effects of screening on disease-specific mortality.
As described previously,3 participants randomized to the screening arm of the PLCO Cancer Screening Trial underwent screening examinations for the detection of prostate (prostate-specific antigen, digital rectal examination), lung (chest x-ray), colorectal (sigmoidoscopy), and ovarian cancer (CA-125; transvaginal ultrasound). Study participants providing annual blood samples (for 6 years) for prostate-specific antigen (men) or CA-125 (women) testing were also asked to provide additional blood samples for research purposes. At baseline, study participants provided written, informed consent in accordance with the Declaration of Helsinki and completed a demographic and risk factor questionnaire.8 Information on incident cancers (type and date) was also collected prospectively using standardized questionnaires by mail to all study participants on an annual basis. For all reported cancers, trained PLCO data abstracters reviewed available clinical records and confirmed each case.
The study population for this investigation was drawn from the 77 469 participants in the screening arm. First, we identified a total of 129 incident CLL cases in the database; 123 provided their consent to participate in research studies. Of these, 109 persons had available prediagnostic serum samples, and they were defined as study subjects. Forty-five of the 109 (41%) persons with available prediagnostic serum had also available stored peripheral whole blood. Those 45 subjects were included in a recent study in which we found 44 (98%; 95% CI, 88%-100%) to have evidence of circulating monoclonal B cells detectable in stored peripheral blood obtained up to 6.4 years before CLL diagnosis.3 In that study, we defined the tumor cell light chain restriction (immunophenotype) in 37 (82%) cases. In addition, we were able to define the IGHV mutational status for 34 cases (76%); 8 of 34 (24%) were unmutated and 26 of 34 (76%) were mutated. In the present investigation, we conducted subanalyses, including information on immunophenotype and IGHV mutational status of the prediagnostic clone.
This study was conducted according to a protocol approved by the Institutional Review Boards of the National Cancer Institute and the 10 screening centers.
Serum protein assays
All serum samples were processed and analyzed in an identical fashion in the Mayo Clinic Protein Immunology Laboratory (Rochester, MN).9 Electrophoresis was performed on agarose gel (REP, Helena Laboratories). The agarose strip was inspected by 2 technicians and by 2 of the authors (R.A.K. and J.A.K.) for restricted migration suggestive of a monoclonal gammopathy and also for polyclonal hypergammaglobulinemia and hypogammaglobulinemia. Hypergammaglobulinemia and hypogammaglobulinemia were defined as γ fractions more than 1.6 g/dL and less than 0.6 g/dL, respectively. In parallel, we assessed all serum samples using immunofixation (Hydrasys and Hydragel, Sebia).10
Concentrations of FLC were determined in all study samples using the FLC assay (Freelite; The Binding Site) performed on a Dade-Behring Nephelometer.10-12 It consists of 2 separate measurements: 1 to quantitate κ FLC (reference range, 0.33-1.94 mg/dL) and 1 for λ FLC (reference range, 0.57-2.63 mg/dL).13 In addition to measuring the concentration of FLCs, the test also provides the κ/λ FLC ratio (reference range, 0.26-1.65).10 Subjects with a κ/λ FLC ratio less than 0.26 are presumed to synthesizing excess λ FLC and those with ratios greater than 1.65 are defined as having excess κ FLC. If the FLC ratio is greater than 1.65, κ is considered to be the “involved” FLC and λ is considered to be the “uninvolved” FLC, and vice versa if the ratio is less than 0.26.
Statistical analysis
We estimated the prevalence of abnormal FLC ratio, M-protein, and hypogammaglobulinemia, dividing the number of persons affected by the total number of persons at risk. Exact 95% binomial CIs were computed for estimates of proportions. We also explored prevalence patterns by sex and age of CLL diagnosis (> 70 or < 70 years). Linear regression models were used to test for trends of the prevalence odds with regard to having protein abnormalities over time. We used generalized estimation equations to take into account the correlations between observations on the same person.14 We used the autocorrelation working correlation matrices in the calculations; other working correlations yielded similar results, however. Furthermore, using descriptive statistics, we evaluated variations in M-protein, FLC, and γ-globulin levels in individual patients.
In a subanalysis restricted to patients with available information on tumor cell light-chain restriction (immunophenotype; n = 37) and IGHV mutational status (n = 34, from our recent study3 ), respectively, we assessed patterns of serum protein markers by immunophenotype and IGHV mutational status. Finally, in a subanalysis restricted to persons with a normal FLC ratio, we estimated the prevalence of elevated free κ (> 1.94 mg/dL) and λ (> 2.63 mg/dL) FLCs.13 Among these subjects, we also defined the median γ-globulin concentration (g/dL) annually up to 10 years before CLL diagnosis.
Results
A total of 109 CLL patients (median age, 70 years; range, 57-80 years; 61% males) with stored prediagnostic serum samples obtained up to 9.8 years before CLL diagnosis were included in the study (Table 1). The majority of study subjects had at least 4 (range, 1-6) available prediagnostic serum samples. The median time between the first and the last prediagnostic blood draw in relation to a subsequent CLL diagnosis was 4.5 years (range, 0.04-10.2 years) and 1.8 years (range, 0.01-8.9 years), respectively.
Patient characteristics
| Variable . | Value . |
|---|---|
| Median age at CLL diagnosis, y (range) | 70 (57-80) |
| Sex, n (%) | |
| Male | 67 (61) |
| Female | 37 (34) |
| Data missing | 5 (5) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 98 (90) |
| Black, non-Hispanic | 3 (3) |
| Asian | 2 (2) |
| Pacific Islander | 1 (1) |
| Data missing | 5 (5) |
| IGHV mutational status,* n (%) | |
| Unmutated | 8 (7) |
| Mutated | 26 (24) |
| Unknown/missing | 75 (69) |
| No. of available annually collected prediagnostic serum samples (per patient), n (%) | |
| 1 or 2 | 31 (29) |
| 3 or 4 | 41 (37) |
| 5 or 6 | 37 (34) |
| Median latency between blood draw and CLL diagnosis, y (range) | |
| First prediagnostic blood draw | 4.5 (0.04-10.2) |
| Last prediagnostic blood draw | 1.8 (0.01-8.9) |
| Variable . | Value . |
|---|---|
| Median age at CLL diagnosis, y (range) | 70 (57-80) |
| Sex, n (%) | |
| Male | 67 (61) |
| Female | 37 (34) |
| Data missing | 5 (5) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 98 (90) |
| Black, non-Hispanic | 3 (3) |
| Asian | 2 (2) |
| Pacific Islander | 1 (1) |
| Data missing | 5 (5) |
| IGHV mutational status,* n (%) | |
| Unmutated | 8 (7) |
| Mutated | 26 (24) |
| Unknown/missing | 75 (69) |
| No. of available annually collected prediagnostic serum samples (per patient), n (%) | |
| 1 or 2 | 31 (29) |
| 3 or 4 | 41 (37) |
| 5 or 6 | 37 (34) |
| Median latency between blood draw and CLL diagnosis, y (range) | |
| First prediagnostic blood draw | 4.5 (0.04-10.2) |
| Last prediagnostic blood draw | 1.8 (0.01-8.9) |
IGHV mutational status defined in monoclonal B cells detected before CLL diagnosis.3
FLCs
We took 2 approaches with regard to our interpretation of the FLC assay results. First, as a measure of monoclonality, we assessed the presence of an abnormal κ/λ FLC ratio. Second, among subjects with a normal κ/λ FLC ratio and without an M-protein, we evaluated patterns of elevated κ and/or λ FLCs as a measure of B-cell activation.
Abnormal κ/λ FLC ratio as a measure of monoclonality
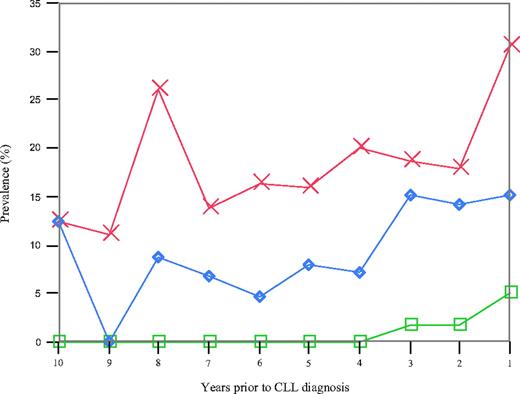
We found 41 of 109 (38%) patients to have an abnormal κ/λ FLC ratio with the earliest detection 9.8 years before CLL diagnosis.15 Among the 41 patients with an abnormal FLC ratio present before CLL diagnosis, 6 (15%) had a detectable M-protein and 1 (2%) had evidence of a concomitant hypogammaglobulinemia. When we estimated the year-by-year prevalence of an abnormal FLC ratio before CLL diagnosis (Figure 1), up to 31% of the CLL patients were found to have an abnormal FLC ratio at one given time point (Figure 1). There was no trend for an increased prevalence of abnormal FLC ratios before the diagnosis of CLL (P trend = .255). The year-by-year prevalence of an abnormal FLC ratio did not vary by sex, age at CLL diagnosis, or mutational status of the tumor cells (data not shown). Patient characteristics were similar for CLL patients with one versus multiple samples exhibiting an abnormal FLC ratio (data not shown).
Year-by-year prevalence of an abnormal FLC ratio (red), a monoclonal protein (blue), and hypogammaglobulinemia (green) among all 109 CLL patients.
Year-by-year prevalence of an abnormal FLC ratio (red), a monoclonal protein (blue), and hypogammaglobulinemia (green) among all 109 CLL patients.
In a subanalysis restricted to patients with information regarding tumor cell immunophenotype (n = 37), we found an association (P = .024, Fisher exact test) between immunophenotype and the involved FLC (Table 2). In 26 (70%) patients with a κ immunophenotype, 9 had an involved κ FLC and 17 had a normal FLC ratio. Among 8 persons with a λ immunophenotype, one had an involved λ FLC, 6 had a normal FLC ratio, and there was one with an involved κ FLC.
Association between tumor cell immunophenotype and involved FLC*
| Tumor cell immunophenotype* . | Normal FLC ratio, n (%) . | Abnormal FLC ratio, n (%) . | ||||
|---|---|---|---|---|---|---|
| Both normal . | Elevated κ . | Elevated λ . | Both elevated . | Involved κ . | Involved λ . | |
| κ (n = 26) | 9 (35) | 6 (23) | 0 (0) | 2 (7) | 9 (35) | 0 (0) |
| λ (n = 8) | 5 (63) | 0 | 1 (13) | 0 | 1 (13) | 1 (13) |
| Biclonal (n = 3) | 0 (0) | 1 (33) | 0 (0) | 1 (33) | 1 (33) | 0 (0) |
| Tumor cell immunophenotype* . | Normal FLC ratio, n (%) . | Abnormal FLC ratio, n (%) . | ||||
|---|---|---|---|---|---|---|
| Both normal . | Elevated κ . | Elevated λ . | Both elevated . | Involved κ . | Involved λ . | |
| κ (n = 26) | 9 (35) | 6 (23) | 0 (0) | 2 (7) | 9 (35) | 0 (0) |
| λ (n = 8) | 5 (63) | 0 | 1 (13) | 0 | 1 (13) | 1 (13) |
| Biclonal (n = 3) | 0 (0) | 1 (33) | 0 (0) | 1 (33) | 1 (33) | 0 (0) |
Percentages do not add up to 100 because of rounding of small numbers. P = .024 (Fisher exact test).
Immunophenotype defined in monoclonal B cells detected before CLL diagnosis.3
Elevated κ and/or λ FLC levels as a measure of B-cell activation
When we estimated the prevalence of elevated κ (ie, > 1.94 mg/dL) and/or λ (ie, > 2.63 mg/dL) FLC, 44 of 109 (40%) patients had elevated κ and/or λ FLC levels before CLL diagnosis (Table 3).16 The prevalence of elevated FLC levels increased significantly with time closer to CLL diagnosis (P trend = .038). In a subanalysis restricted to persons with a normal FLC ratio and without an M-protein (n = 61), 17 had elevated κ and/or λ FLC levels, indicating polyclonal B-cell activation in 17 of 109 (16%; Table 2). Patterns of elevated FLC levels among persons with a normal FLC ratio were found up to 9 years before CLL diagnosis.
Year-by-year prevalence of elevated free κ FLC, free λ FLC, or both
| . | Patients with a normal FLC ratio and without an M-protein (n = 61) . | All patients (n = 109) . | ||||
|---|---|---|---|---|---|---|
| N . | n . | Percentage (95% CI) . | N . | n . | Percentage (95% CI) . | |
| Blood draw before CLL diagnosis, y | ||||||
| 1 | 30 | 4 | 13 (4-31) | 59 | 20 | 34 (22-47) |
| 2 | 33 | 7 | 21 (9-39) | 56 | 17 | 30 (19-44) |
| 3 | 29 | 7 | 24 (10-44) | 59 | 21 | 36 (24-49) |
| 4 | 33 | 6 | 18 (7-35) | 55 | 16 | 29 (18-43) |
| 5 | 28 | 6 | 21 (8-41) | 50 | 19 | 38 (25-53) |
| 6 | 24 | 6 | 25 (10-47) | 43 | 14 | 33 (19-49) |
| 7 | 16 | 2 | 13 (2-38) | 29 | 7 | 24 (10-44) |
| 8 | 13 | 1 | 8 (0.2-36) | 23 | 6 | 26 (10-48) |
| 9 | 7 | 1 | 14 (0.4-58) | 9 | 1 | 11 (0.3-48) |
| 10 | 7 | 0 | 0 | 8 | 1 | 13 (0.3-53) |
| P trend | .145 | .038 | ||||
| . | Patients with a normal FLC ratio and without an M-protein (n = 61) . | All patients (n = 109) . | ||||
|---|---|---|---|---|---|---|
| N . | n . | Percentage (95% CI) . | N . | n . | Percentage (95% CI) . | |
| Blood draw before CLL diagnosis, y | ||||||
| 1 | 30 | 4 | 13 (4-31) | 59 | 20 | 34 (22-47) |
| 2 | 33 | 7 | 21 (9-39) | 56 | 17 | 30 (19-44) |
| 3 | 29 | 7 | 24 (10-44) | 59 | 21 | 36 (24-49) |
| 4 | 33 | 6 | 18 (7-35) | 55 | 16 | 29 (18-43) |
| 5 | 28 | 6 | 21 (8-41) | 50 | 19 | 38 (25-53) |
| 6 | 24 | 6 | 25 (10-47) | 43 | 14 | 33 (19-49) |
| 7 | 16 | 2 | 13 (2-38) | 29 | 7 | 24 (10-44) |
| 8 | 13 | 1 | 8 (0.2-36) | 23 | 6 | 26 (10-48) |
| 9 | 7 | 1 | 14 (0.4-58) | 9 | 1 | 11 (0.3-48) |
| 10 | 7 | 0 | 0 | 8 | 1 | 13 (0.3-53) |
| P trend | .145 | .038 | ||||
N indicates number of participants with blood draw at each time point in relation to the diagnosis of CLL; and n, number of participants with elevated levels of free κ FLC (defined as more than 1.94 mg/dL), elevated levels of free λ FLC (defined as more than 2.63 mg/dL), or both.
Monoclonal serum proteins
There were a total of 14 of 109 (13%) patients with an M-protein before the diagnosis of CLL (Figure 1), including 6 with a concomitant abnormal FLC ratio. There was no trend of an increased prevalence of M-proteins before CLL diagnosis (P trend = .14; Figure 1). Among the 14 patients with an M-protein, 4 were IgG, 7 were IgM, and 3 had a biclonal isotype. With regard to the light chain patterns, there were 10, 3, and 1 patients with a κ, λ, and biclonal isotype, respectively (Table 4). None of the 14 patients had hypogammaglobulinemia before CLL diagnosis. An M-protein was first detectable in prediagnostic blood collected 9.8 years before the diagnosis of CLL in 1 patient who also had an abnormal FLC ratio.
Isotypes of heavy and light chains among 14 patients with an M-protein
| Variable . | No. (%) . |
|---|---|
| Total patients | 14 (100) |
| Isotype of heavy chain | |
| IgG | 4 (29) |
| IgA | 0 (0) |
| IgM | 7 (50) |
| Biclonal | 3 (21) |
| Isotype of light chain | |
| κ | 10 (71) |
| λ | 3 (21) |
| Biclonal | 1 (7) |
| Variable . | No. (%) . |
|---|---|
| Total patients | 14 (100) |
| Isotype of heavy chain | |
| IgG | 4 (29) |
| IgA | 0 (0) |
| IgM | 7 (50) |
| Biclonal | 3 (21) |
| Isotype of light chain | |
| κ | 10 (71) |
| λ | 3 (21) |
| Biclonal | 1 (7) |
We found no relationship between prediagnostic M-proteins and mutational status of the clonal cells3 (data not shown). However, based on small numbers, we found that M-proteins were more prevalent (P = .05) among males (15%) than females (3%). Similarly, M-proteins were more prevalent among younger (< 70 years) compared with older (> 70 years) persons diagnosed with CLL (P = .067).
γ-Globulins
We found that 3 of 109 (3%) CLL patients had hypogammaglobulinemia, starting from 3 years before CLL diagnosis (Figure 1). Two of these patients also had an abnormal FLC ratio. To further explore evidence of B-cell activation among persons with a normal FLC ratio and without an M-protein, we assessed patterns of γ-globulin concentration before the diagnosis of CLL. The median γ-globulin levels were stable over time, ranging from 1.0 to 1.2 g/dL. We found 11 subjects with evidence of hypergammaglobulinemia (range of γ-globulin concentration, 1.63-4.79 g/dL) before the diagnosis of CLL.
Discussion
In this first large population-based screening study based on 109 CLL patients with available stored prediagnostic blood samples up to 9.8 years before CLL diagnosis, we found 38% and 13% of the patients to have an abnormal FLC ratio and an M-protein, respectively. These rates are substantially higher than the prevalence of abnormal FLC ratios and M-proteins in the general population 50 years or older (reportedly ∼ 2%-3%).9,17
Although traditionally thought to be derived from naive B cells, indeed, recent studies1,18-20 support the derivation of CLL from activated, antigen-experienced B cells. In accord with this theory, population-based studies from the United States21 and Scandinavia22 have suggested that respiratory tract infections may trigger the development of CLL. However, it remains unclear whether protein abnormalities present before CLL diagnosis are triggered by some infectious agent or they are consequences of an underlying immune defects, which is manifested later by various infections. To further address this issue, in the present study, we assessed patterns of tumor cell immunophenotype and involved FLCs and found a strong association (P = .024, Fisher exact test). Indeed, with the exception of one case, all patients with an involved κ FLC had either κ immunophenotype or they were biclonal; all patients with an involved λ FLC had either λ immunophenotype or they were biclonal (Table 2). Based on these patterns, we have speculated that the observed FLCs and M-proteins might be byproducts of the CLL cells because of their turnover.23-25
Furthermore, among persons with a normal FLC ratio and without an M-protein (n = 61), there were 17 (28%) with elevated free κ and/or λ FLC levels, indicating polyclonal B-cell activation. On the other hand, study reported elevated free κ or λ FLC levels, presenting by an extended FLC ratio 0.37 to 3.1, could also be the result of impaired renal function with the existence of low level of multiple myeloma.26 However, given there is no increased frequency of renal disease in CLL and the base population in the PLCO trial is healthy, we think that renal dysfunction is unlikely to influence the observed elevated κ and/or λ FLC levels in this study. Taken together, our findings support a role for chronic immune stimulation in the etiology of CLL. Future research with longitudinal follow-up of persons with protein abnormalities, with or without monoclonal B-cell lymphocytosis, is needed to further define these processes and their precise contribution to CLL development.
In sharp contrast to the observed prevalence of prediagnostic abnormal FLC ratios and M-protein patterns, we found only 3% of the CLL patients to have prediagnostic hypogammaglobulinemia. In one patient, this was detectable up to 3 years before CLL diagnosis. Consistent with prior studies,27 this supports the contention that hypogammaglobulinemia is a late event in CLL progression.
Interestingly, females with CLL have been found to have a more favorable prognostic disease profile; for example, women are diagnosed with less advanced stage and more favorable chromosomal abnormalities than males.28 Recent population-based data show that female CLL patients have a lower excess mortality compared with males.29-31 When we assessed abnormal serum protein patterns by sex, we found that more than 10% of the male patients had an M-protein before CLL diagnosis; however, only one female case had evidence of a prediagnostic M-protein. We also found prediagnostic M-proteins to be more prevalent among CLL patients diagnosed at younger compared with older ages (< 70 or > 70 years). Future studies are needed to explore underlying mechanisms of these associations. The proportions of males and females with an abnormal serum FLC ratio were virtually the same (37% vs 38%).
Strengths of our study include its unique population-based prospective design, available stored prediagnostic blood samples, and the application of high-quality assays for the determination of prediagnostic B-cell clones. Because of the design of the PLCO Cancer Screening Trial, a limitation is the lack of available blood samples at the time of CLL diagnosis.
Our results are timely given previous studies reporting a 38.6% of abnormal serum FLC ratio based on a retrospective analysis of 259 pretreated/treated CLL patients6 and that CLL patients with M-proteins4 and abnormal FLC ratios6 at CLL diagnosis have a poorer survival than those without. Indeed, the most recent study shows that Zap-70 expression, elevated β-2-microglobulin levels, unmutated IGHV genes, and the presence of an abnormal FLC ratio are independent negative prognostic variables for overall survival in CLL.6 In addition, in contrast to, for example, flow cytometry and IGHV gene rearrangement analyses, the protein assays applied in our study are easily available in most standard clinical care settings. Further studies with longitudinal follow-up and larger sample size are needed to explore the role of serum protein abnormalities in relation to cytogenetic abnormalities, such as ZAP-70, CD38 expression, and IGHV in the development of CLL, their applications in the early detection of CLL, as well as in disease progression and outcome.
In conclusion, we found slightly less than 40% of CLL patients to have monoclonal serum protein abnormalities detectable up to 9.8 years before the diagnosis of CLL. Another 16% of the patients had elevated free κ and/or λ FLC levels, which indicates an ongoing polyclonal B-cell activation. Thus, our findings support a role for chronic immune stimulation in the etiology of CLL. Together with our observation that the tumor cell immunophenotype and the involved FLC are associated, it suggests that the CLL clone and the observed serum protein abnormalities originate from the same clone.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Research Grants CA 62242 and CA 107476 from the National Cancer Institute and the Intramural Research Program of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: H.-T.T. and O.L. initiated this work and wrote the report; R.A.K. and J.A.K. (and 2 technicians) inspected all electrophoresis assays; and all authors were involved in the design of the study, obtained and analyzed data, were involved in the interpretation of the results, read, gave comments, and approved the final version of the manuscript, had full access to the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: M. A. is an employee of Quest Diagnostics, Nichols Institute, San Juan Capistrano, CA. The remaining authors declare no competing financial interests.
Correspondence: Ola Landgren, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, 9000 Rockville Pike, Bldg 10/Rm 13N240F, Bethesda, MD 20892; e-mail: landgreo@mail.nih.gov.