Abstract
Allogeneic hematopoietic stem cell transplantation is an established treatment modality for malignant and nonmalignant hematologic diseases. Acute and chronic graft-versus-host diseases (GVHDs) are a major cause of morbidity and mortality after allogeneic stem cell transplantation. T cells have been identified as key players in the graft-versus-host reaction and, therefore, most established drugs used against GVHD target T cells. Despite our knowledge on the pathogenesis of the GVH reaction, success of established therapies for prevention and treatment of GHVD is unsatisfactory. Recently, animal and human studies demonstrated that B cells are involved in the immunopathophysiology of acute and chronic GVHD. Early phase clinical trials of B-cell depletion with rituximab have shown beneficial effects on both acute and chronic GVHD. This review summarizes the current experimental and clinical evidence for the involvement of B cells in the pathogenesis of acute and chronic GVHD and discusses the clinical implications for the management of patients undergoing allogeneic stem cell transplantation.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an established, potentially curative treatment modality for malignant and nonmalignant hematologic diseases. Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality after allogeneic HSCT and limits its wider use. Traditionally, GVHD has been divided into 2 forms, acute and chronic, based on the time of its onset. Acute GVHD has been defined as disease occurring in the first 100 days after transplantation, whereas chronic GVHD occurs after day 100. This arbitrary distinction based on the time of onset fails to reflect the different pathophysiologic mechanisms and clinical manifestations of acute and chronic GVHD, however. Acute GVHD can occur after day 100 in patients who received a nonmyeloablative conditioning regimen or donor lymphocyte infusions. In addition, GVHD with typical clinical features of chronic GVHD can develop well before day 100 and concurrent with acute GVHD. Therefore, the National Institutes of Health consensus development project has defined new criteria for the diagnosis, staging, and response assessment of chronic GVHD.1,2 Only a few effective therapies are currently available for the treatment of both forms of GVHD. The established immunosuppressive agents are mostly nonspecific and unfortunately associated with severe side effects, in particular the susceptibility to life-threatening infections. Therefore, novel more selective agents are urgently needed.
GVHD is an immune-mediated disease that results from a complex interaction between donor and recipient adaptive immunity. Donor-derived CD4+ and CD8+ T lymphocytes have classically been considered to be the main effector cells mediating GVHD pathogenesis. In fact, removal of T cells from transplant inocula almost completely prevents GVHD from developing, however, at the price of increased incidences of graft rejection and disease recurrence. In recent years, basic and clinical research has provided a more detailed mechanistic understanding of the molecules and cell types involved in the biology of the graft-versus-host reaction.
Because acute GVHD is thought to be mediated mainly by donor T cells, preventive and therapeutic treatment strategies have focused primarily on the inhibition of T-cell function. Recent animal studies suggest that B cells might also play an important role in the biology of GVHD. Further circumstantial evidence for the involvement of B cells in GVHD pathogenesis comes from reports of successful treatment of GVHD with B-cell depletion. The mechanisms by which B cells contribute to acute and chronic GVHD currently are only incompletely understood. Here, we provide an overview of the experimental and clinical evidence for a pathogenic role of B cells in GVHD and evaluate the implications with regard to B cell–targeted therapies for the treatment of GVHD. We conclude by providing an outlook on novel B cell–specific approaches that might prove beneficial for the treatment of GVHD.
B-cell functions in health and disease
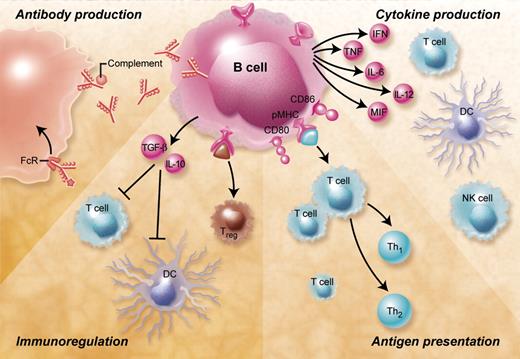
B cells are central players of the humoral immune response (Figure 1). They are specialized in the production of antibodies and thereby provide a protective immune defense against bacteria, viruses, and harmful protein antigens such as toxins. After binding to their target antigen antibodies can lead to complement activation, antibody-mediated cellular cytotoxicity, immune complex formation, and Fc-mediated endocytosis. Over the last decade this view, which focused on the importance of B cells for the humoral immune response, has changed dramatically. Accumulating evidence suggests that apart from antibody production B cells contribute to the immune response by important antibody-independent mechanisms such as presentation of antigen, the production of cytokines and chemokines, as well as by acting as immunoregulatory cells.
Overview of the B-cell functions and their potential relevance for GVHD. B cells contribute to immune responses by antibody-mediated and antibody-independent mechanisms. Antibodies produced by B cells can lead to complement activation, antibody-dependent cell-mediated cytotoxicity, and Fc-receptor antigen uptake and phagocytosis. B cells can furthermore secrete a large number of proinflammatory cytokines including IL-2, TNF-α, IL-6, IL-12, MIF, and interferon-γ that activate a large number of immune cells such as T cells (including Th17 cells), macrophages, and natural killer (NK) cells and have been shown to be involved in regulation of the GVH reaction. Antigen presentation by activated B cells that have up-regulated major histocompatibility complex and costimulatory molecules such as CD80 and CD86 leads to CD4+ and CD8+ T-cell activation and differentiation. Depending on the B-cell subset and the nature of the activation stimulus B lymphocytes can also act as immunoregulatory cells that induce peripheral CD4+ and CD8+ T-cell tolerance, inhibit dendritic cells, and induce and expand regulatory T cells.
Overview of the B-cell functions and their potential relevance for GVHD. B cells contribute to immune responses by antibody-mediated and antibody-independent mechanisms. Antibodies produced by B cells can lead to complement activation, antibody-dependent cell-mediated cytotoxicity, and Fc-receptor antigen uptake and phagocytosis. B cells can furthermore secrete a large number of proinflammatory cytokines including IL-2, TNF-α, IL-6, IL-12, MIF, and interferon-γ that activate a large number of immune cells such as T cells (including Th17 cells), macrophages, and natural killer (NK) cells and have been shown to be involved in regulation of the GVH reaction. Antigen presentation by activated B cells that have up-regulated major histocompatibility complex and costimulatory molecules such as CD80 and CD86 leads to CD4+ and CD8+ T-cell activation and differentiation. Depending on the B-cell subset and the nature of the activation stimulus B lymphocytes can also act as immunoregulatory cells that induce peripheral CD4+ and CD8+ T-cell tolerance, inhibit dendritic cells, and induce and expand regulatory T cells.
Antigen presentation by B cells plays a fundamental role during physiologic immune responses. After activation via the B-cell receptor (BCR) and costimulatory receptors such as CD40, B lymphocytes become potent antigen-presenting cells (APCs).3 What sets B cells apart from other APCs is their unique ability of antigen-specific antigen uptake through the BCR. After binding to the BCR B lymphocytes internalize antigen and the internalized antigens are subsequently processed and presented in the context of major histocompatibility complex (MHC) class II as well as cross-presented through MHC class I.4 Activated B cells can prime both CD4+ and CD8+ T cells in vivo3,5 and antigen presentation by B cells is important in determining the level of T-cell responses. This was revealed by experiments with mice that lack MHC class II specifically on B cells but not on dendritic cells (DCs), which were characterized by impaired expansion and differentiation of T cells after immunization.6 In addition to presenting antigen B lymphocytes can generate and secrete several cytokines and chemokines. They can thereby shape the type and strength of the immune response by activation and recruitment of other immune cells. For instance, analogous to the CD4+ effector T-cell subsets Harris et al have identified 2 functionally distinct B-cell subsets, termed B effector 1 and B effector 2 cells, that differentially regulate T-cell responses by the production of different sets of cytokines.7
In several autoimmune diseases antibody-independent B-cell functions play an important role. More than half of the of the developing B cells in the bone marrow express autoreactive B-cell receptors (BCRs).8 These autoreactive B cells are usually deleted or anergized but failure of tolerance checkpoints can lead to escape from deletion of self-reactive B cells and the production of autoantibodies and autoimmune disease. In patients with autoimmune diseases such as systemic lupus erythematosus (SLE) high levels of B cell–trophic factors such as B cell–activating factor of the tumor necrosis factor (TNF) family (BAFF), also known as B-lymphocyte stimulator, can be found and suggest that B lymphocytes participate in the autoimmune disease process. BAFF provides survival signals to B cells and protects them from apoptosis by binding to 3 TNF receptor family members, B-cell maturation antigen, transmembrane activator and calcium modulator and cyclophilin ligand interactor, and B-lymphocyte stimulator receptor 3 (BR3, also known as BAFF receptor). Overexpression of BAFF in mice leads to the development of autoimmune disease,9 whereas neutralization of BAFF results in amelioration of autoimmune pathology.10 Activated B lymphocytes secrete a variety of proinflammatory cytokines and chemokines, for example, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-12, and macrophage migration inhibitory factor (MIF), which have been implicated in participating in the inflammatory cascade of autoimmune pathology. In addition, antigen presentation by autoreactive B cells was found to be critical in several autoimmune disorders, for example, SLE or rheumatoid arthritis, and can promote autoimmune pathology independent of antibody production.11-13
In contrast to their immunostimulatory characteristics, certain B-cell subsets can also exercise an immunomodulatory function. They maintain peripheral tolerance of CD4+ and CD8+ T cells by a variety of mechanisms, such as deletion,14 induction of anergy,15 and cytokine-mediated suppression.16 For instance, antigen presented by resting B cells leads to only partial activation of T cells and results in T-cell tolerance.17 Consistent with a tolerogenic role of B cells the immunologic response to tumor vaccination is enhanced in the absence of B cells.18 How regulatory B cells mediate suppression in not fully understood. The tolerogenic properties of resting B cells may be due to the low expression of costimulatory molecules. As outlined before, activation of B cells via CD40 generates antigen-presenting B cells with strong stimulatory capacity. Stimulation with certain other stimuli such as lipopolysaccharide or Staphylococcus aureus Cowan I, on the other hand, renders B cells tolerogenic, despite inducing up-regulation of costimulatory molecules.16,19 Regulatory B cells induce tolerance not only by acting as tolerogenic APCs, but also by producing well-known immunomodulatory cytokines, foremost IL-10 and TGF-β, which can suppress T-cell responses.16,20
In addition their direct effects on T and B cells have been shown to regulate the T-cell response indirectly by suppressing a broad range of other immune cells. They possess the ability to thwart T-cell responses by inhibiting DCs.21,22 After vaccination DCs from B cell–deficient μMT mice secrete higher amounts of IL-12 than DCs from wild-type mice. B lymphocytes can furthermore attract regulatory T cells23 and have been shown to induce and expand murine and human regulatory T cells in vitro.24,25 A functional role for immunoregulatory B cells in preventing autoimmune disease was first revealed in studies of murine experimental allergic encephalitis, an animal model for multiple sclerosis. In this model the inhibitory effect of B cells is dependent on IL-10 produced by a rare subpopulation of CD1dhigh CD5+ regulatory B cells.26,27 The case of experimental allergic encephalitis also exemplifies that B lymphocytes can exert opposing functions depending on the subset and phase of the disease course. Regulatory B cells protect against the development of experimental allergic encephalitis, whereas B-cell depletion after onset of disease ameliorates disease progression.27 A regulatory function of B cells has also been confirmed in animal models of rheumatoid arthritis and inflammatory bowel disease.20 Taken together, recent knowledge of B-cell biology has revealed that B cells possess considerable functional heterogeneity. The type of B-cell subsets involved, the nature, strength, and duration of the activation stimulus, as well as the temporospatial context determine whether B cells exert stimulatory or inhibitory effects on the immune system.
The advent of B cell–depleting monoclonal antibodies made it possible to study the importance of B cells in human disease. Data from clinical trials of rituximab in patients with autoimmune diseases have provided important insights into the involvement of B cells in many aspects of the autoimmune reaction. Rituximab is a chimeric monoclonal antibody directed against the B cell–specific cluster of differentiation 20 (CD20) antigen, a transmembrane protein expressed on pre-B lymphocytes, and immature and mature B cells but not on early B-cell precursors or plasma cells. It was initially developed as a treatment against B-cell lymphomas. Rituximab binds to CD20 on B lymphocytes and malignant lymphoma and causes cell destruction by a multifactorial mechanism including antibody-dependent cell-mediated cytotoxicity, complement-mediated cell lysis, and direct induction of apoptosis of target cells. Administration of rituximab leads to a rapid and selective depletion of B cells that lasts for several months. Because plasma cells do not express CD20, serum immunoglobulin levels are usually not affected by treatment with rituximab.
Based on the experimental evidence for a pathogenic role of B cells in autoimmune disease and reports of improvement of autoimmune manifestations in lymphoma patients who were treated with rituximab, B-cell depletion has been investigated as a treatment for many autoimmune diseases. Interestingly, it has shown effectiveness even in autoimmune diseases that previously have been thought to be mainly T cell–mediated.28 Meanwhile, due to its effectiveness rituximab has already been approved for the treatment of patients with rheumatoid arthritis who do not respond to TNF blockade. The clinical benefit of rituximab in autoimmune disease generally correlates with the extent and duration of B-cell depletion. Individual clinical responses do not always correlate with changes in autoantibody serum levels, however. Treatment with rituximab not only depletes B cells but also down-regulates the expression of important costimulatory molecules such as CD40 and CD80, possibly leading to diminished T-cell activation.29 Moreover, after rituximab treatment the number and suppressive function of regulatory T cells increases.30 In addition to short-term depletion of B lymphocytes rituximab also has beneficial long-term effects that persist even after recovery of the B-cell compartment. It appears that rituximab reverses some of the disturbances of the peripheral B-cell pool and reconstitutes a normal B- and T-cell homeostasis in patients with autoimmune disease.31
B-cell reconstitution kinetics after allogeneic HSCT
After myeloablative allogeneic HSCT there is a long-lasting defect of cell-mediated immunity. A variety of factors such as the type and intensity of conditioning, stem cell source, drugs used for immunosuppression, and occurrence of infection or GVHD influences the kinetics of immune recovery. B-cell reconstitution is generally slow and immunoglobulin levels are reduced after transplantation. In some patients immunoglobulin levels remain decreased for more than a year. Consequently, transplant recipients are at an increased risk of infection especially to encapsulated bacteria due to low levels of immunoglobulin A (IgA) and IgG2 and slower B-cell reconstitution is associated with higher infection rates.32 The reconstitution of B cells after transplantation seems to recapitulate B-cell ontogeny.33,34 Early after transplantation there is a restricted B-cell repertoire, with limited BCR diversity that recovers only slowly.34 Furthermore, even despite quantitative normalization of the B-cell pool within the first year after transplantation, these B cells are functionally impaired.33 They exhibit a defect in the acquisition of somatic mutations and respond poorly to antigen, especially polysaccharide antigens.35
Several factors affect the recovery of B cells after allogeneic stem cell transplantation. The B-cell repopulation is substantially influenced by the type of stem cells and the graft content. Peripheral blood stem cell grafts contain more B and T cells than bone marrow stem cell grafts. They have 3 to 18 times more B cells than bone marrow stem grafts resulting in a faster early B-cell recovery.36,37 Possibly due to stem cell mobilization with granulocyte colony-stimulating factor blood stem cell grafts also contain a higher proportion of B cells with an activated phenotype.38 The occurrence of GVHD can also have a profound influence on B-cell recovery. Patients experiencing acute or chronic GVHD have lower B-cell numbers that could be due to GVHD itself or its treatment.39,40 Both acute and chronic GVHD are accompanied by a lower B-cell generation from B-cell progenitors in the bone marrow.39,41 In addition, donor T cells can contribute to GVHD-associated B-cell deficiency by Fas ligand–mediated cytotoxicity or release of cytokines such as interferon-gamma, which inhibit B-cell lymphopoiesis.42,43 Lack of T-cell help and lymphoid hypoplasia in the posttransplantation period might further contribute to the slow recovery of B cells. Obviously, the type and duration of immunosuppression likewise affect the dynamics of B-cell recovery and prolonged immunosuppression correlates with a slower B-cell reconstitution.44
Data on the B-cell reconstitution after nonmyeloablative conditioning regimens are scarce and contradictory. Whereas one study reported a higher total B-cell count after nonmyeloablative conditioning,45 another study observed a delayed B-cell recovery.46 After reduced-intensity conditioning host B cells persist for prolonged times and the patients have higher numbers of memory B cells.45 As a consequence, humoral immunity to viral and bacterial pathogens such as Epstein-Barr virus, varicella zoster virus, and pneumococci persists longer after reduced-intensity conditioning.47
B cells and acute GVHD
Acute GVHD is a complex disease process resulting in donor T cell–mediated destruction of host tissues. The mainstay first-line therapy is glucocorticoids. Patients who develop steroid-refractory acute GVHD have a very poor prognosis with long-term survival of 25% to 35%. T cells and antigen-presenting cells play an essential role in the immunopathogenesis of GVHD. Insights from murine models have enabled the definition of discrete stages in the evolution of acute GVHD. In the initiating step of acute GVHD the conditioning regimen leads to tissue damage causing the release of proinflammatory cytokines. The inflammatory posttransplantation milieu subsequently promotes the activation of host APCs, which survive the conditioning regimen.48 However, in humans GVHD has also been seen after minimal intensity regimens and after donor lymphocytes infusion (DLI) without conditioning, suggesting that a cytokine storm is not necessarily required for GVHD to develop. The second stage is characterized by the activation of donor alloreactive T cells. The activated host APCs present alloantigen and stimulate alloreactive donor T cells contained in the stem cell graft. After recognition of the foreign host antigens in the context of the MHC on host antigen-presenting cells donor T cells begin to proliferate and differentiate into effector cells. Host APCs are essential and sufficient for the initiation of acute GVHD.49,50 Although donor APCs are not required for the development of the GVH reaction donor APCs can augment acute GVHD.51 Moreover, the type and location of APCs determines the extent and distribution of organ damage.48,52 In the effector stage T cell–induced tissue destruction triggers further inflammation and recruitment of additional effector cells resulting in a further amplification of the inflammatory disease process and tissue damage. The main organs targeted in acute GVHD are skin, gastrointestinal tract, and liver.
Little is known about the involvement of B cells in the pathogenesis of acute GVHD. In mice, B-cell depletion results in a decreased incidence of acute GVHD.53 Additional support for a role of B cells in acute GVHD comes from several recent clinical observations. Encouraged by reports of the effectiveness of B-cell depletion in the treatment of chronic GVHD, Kamble et al treated patients with acute GVHD who were refractory to multiple immunosuppressants with rituximab.54 Three patients who received rituximab showed a complete response of their acute GVHD. Results from recent studies indicate that B-cell depletion might also be effective for the prevention of acute GVHD. Rituximab administered as part of a myeloablative or nonmyeloablative conditioning regimen or given shortly before or after transplantation results in lower-than-expected rates of GVHD.55-59 However, contrary to these studies posttransplantation administration of rituximab did not reduce the incidence of GVHD in a trial of allogeneic stem cell transplantation in patients with a high risk of relapse of aggressive B-cell non-Hodgkin lymphoma.60 It therefore seems that the timing of rituximab administration is a relevant factor and that early B-cell depletion is crucial. Importantly, rituximab administered before or shortly after transplantation appears safe and does not interfere with engraftment.61 Not unexpectedly, B-cell recovery is delayed, however.62 Consistent with a role of donor B cells in acute GVHD, high numbers of B lymphocytes in the apheresis product correlate with an increased incidence of acute GVHD and increased treatment-related mortality.63 Thus, activation of donor B cells contained in the stem cell graft by cognate interaction with alloantigens could result in activation and expansion of alloreactive donor T cells.
On the contrary, even though recipient APCs are essential for the initiation of acute GVHD, host B cells can also have a protective effect. Paradoxically, B cell–deficient mice experience more severe acute GVHD than wild-type mice.64 In this experimental model IL-10 production by recipient B cells is responsible for the suppression of acute GVHD. This is interesting because IL-10 was identified as a critical modulator of acute GVHD.65-68 In humans a high content of B-cell progenitors in the stem cell graft is associated with a significantly lower rate of acute GVHD.69 Whether B cells are involved in acute GVHD is still very controversial but the observation that B cells could play both a pathogenic as well as protective role in acute GVHD could explain the mixed results obtained with B-cell depletion for the prevention and treatment of acute GVHD. A more selective modulation of B cells that target only the pathogenic subset while sparing regulatory B cells could thus result in improved outcomes.
B cells and chronic GVHD
Chronic GVHD is a major cause of morbidity and mortality in long-term survivors of allogeneic hematopoietic stem cell transplantation. Up to 70% of patients experience chronic GVHD. It may develop continuously from acute GVHD, recur after resolution of acute GVHD, or occur de novo. Human leukocyte antigen disparity, source of progenitor cells, graft composition, donor and patient age and sex, and previous acute GVHD have been identified as the main risk factors predicting the development of chronic GVHD. The use of stem cells from peripheral blood is associated with an increased rate of chronic GVHD.70 Chronic GVHD affects a wide range of organs. Among the most commonly affected organs are the skin, liver, gut, lung, and mucous membranes. Chronic GVHD has features resembling autoimmune disorders such as scleroderma, primary biliary cirrhosis, and bronchiolitis obliterans. It can lead to debilitating complications such as joint contractures, blindness, and end-stage lung disease. Glucocorticoids, with or without a calcineurin inhibitor such as ciclosporin or tacrolimus, currently are the standard regimen as primary treatment for chronic GVHD. No standard treatment for steroid-refractory chronic GVHD currently exists.
Several independent lines of evidence clearly demonstrate that B cells are involved in the pathogenesis of chronic GVHD. Zhang et al demonstrated in a minor histocompatibility-mismatched murine model of chronic GVHD that in addition to donor CD4+ T cells donor B cells are required for the induction of chronic GVHD.71 Furthermore, autoantibodies can frequently be detected in patients with chronic GVHD.72-80 Because a considerable number of circulating human class-switched IgG+ memory B cells express autoreactive antibodies, the deregulated inflammation during GVHD might lead to activation of these autoreactive B cells and subsequent autoantibody production. The development of these antibodies requires the presence of alloreactive CD4 T cells81 and the appearance and titer of autoantibodies have been correlated with GVHD onset and activity.72-77 The association of autoantibodies with chronic GVHD is controversial because others found no correlation with disease onset or severity.78-80 After sex-mismatched stem cell transplantation, alloantibodies to Y chromosome–associated minor histocompatibility antigens (H-Y antibodies) can frequently be found and they correlate with the occurrence of chronic GVHD.82,83 These antibodies are derived from donor B cells and are the result of a coordinated response of B and T cells.84,85 Weekly administration of rituximab for 1 month starting 2 months after transplantation prevents the development of anti–H-Y antibodies and seems to protect against the development of chronic GVHD.62,86
Whether the occurrence of antibodies in GVHD represents a bystander effect caused by polyclonal B-cell activation remains unknown. In fact, to date a direct involvement of autoantibodies in GVHD pathology has not been conclusively demonstrated. Transfer of autoantibodies from mice with GVHD to normal mice failed to cause autoimmune pathology.87 On the other hand, stimulatory antibodies to the platelet-derived growth factor receptor that can be found in patients with chronic GVHD or systemic sclerosis but not in patients without GVHD are able to induce an increased production of collagen from human fibroblasts in vitro.75 In light of these findings, autoantibodies could therefore contribute to some features of chronic GVHD such as fibrotic skin changes. But so far, apart from autoantibody-dependent autoimmune phenomena such as immune-mediated hemolysis or thrombocytopenia, no direct evidence for the causal relationship of autoantibodies or alloantibodies and the pathogenesis of organ manifestations of chronic GVHD in humans exists. Instead, the detection of alloreactive and autoreactive antibodies might just serve as a marker for the presence of antigen-specific B cells that contribute to disease pathology by mechanisms other than antibody production. Given the long serum half-life of immunoglobulins and the dynamics of response to therapy with rituximab—responses within 1 to 2 weeks after the start of therapy were observed in some studies—a solely antibody-mediated mechanism of GVHD seems unlikely, however. One interesting experimental approach to answer this question and to distinguish between the antibody-dependent and antibody-independent effects of B cells would be the use of the transgenic mouse strain, which lacks secreted antibodies, used by Chan et al to demonstrate that B cells contribute to SLE pathogenesis in an antibody-independent fashion.11
Chronic GVHD is associated with a perturbed B-cell homeostasis.88 Like patients with autoimmune disease, patients who develop chronic GVHD have a relative reduction in naive B cells and relatively higher numbers of activated memory-type CD27+ B cells.89 Elevated levels of BAFF have been correlated with the development and severity of chronic GVHD.89-91 High levels of BAFF in the presence of lower numbers of naive B cells might foster the survival of activated alloreactive and autoreactive B cells, resulting in immune pathology.89 Interestingly, patients with chronic GVHD had a faster initial reconstitution of the B-cell population.73 In line with the notion that alloreactive donor B cells drive the GVH reaction, a higher content of B cells in the stem cell graft was identified as a risk factor for the occurrence of chronic GVHD.92,93
The incidental clinical observation that treatment of immune thrombocytopenia in a patient with chronic GVHD with rituximab led to improvement of some manifestations of chronic GVHD such as xerophthalmia and enabled discontinuation of immunosuppression raised the question whether the depletion of B cells could be used to treat chronic GVHD.94 Subsequently, the effectiveness of rituximab for the treatment of chronic GVHD was confirmed by several case series and small clinical trials.95-102 Table 1 summarizes the published data. In the largest prospective study of rituximab use for GVHD so far 14 of 20 patients responded to treatment.101 A complete response occurred in 2 patients. Therapy allowed steroid tapering and the median daily dose of prednisolone fell from 40 mg before to 10 mg after treatment with rituximab. In 68% of the patients in this trial, prednisolone could be reduced at least by 50%. Moreover, response to treatment was associated with a decrease of H-Y antibodies in the serum of 4 male patients who received stem cells from female donors. A recent small trial in 13 patients with steroid-refractory GVHD showed that therapy with low-dose (ie, 50 mg/m2) rituximab is feasible and appears to be equally effective as the standard dose of 375 mg/m2 rituximab.103
Summary of published case series and clinical trials of rituximab treatment for chronic GVHD
| Reference(s) . | ORR, no./total no. (%) . | Response site . |
|---|---|---|
| Ratanatharathorn et al95 | 4/8 (50) | Cut, Lu, Ms, O |
| Canninga-van Dijk et al98 | 5/6 (83) | Cut, Li |
| Okamoto et al100 | NA | Cut |
| Zaja et al97 | (65) | Cut, Li, Ms, Lu, Gi, O |
| Cutler et al101 | 14/20 (70) | Cut, Ms, Li |
| Mohty et al99 | 10/15 (66) | Cut, Gi, Li, O |
| von Bonin et al103 | 9/13 (69) | Cut, Ms, O |
| Teshima et al102 | 3/7 (43) | Cut, Ms, O |
| Reference(s) . | ORR, no./total no. (%) . | Response site . |
|---|---|---|
| Ratanatharathorn et al95 | 4/8 (50) | Cut, Lu, Ms, O |
| Canninga-van Dijk et al98 | 5/6 (83) | Cut, Li |
| Okamoto et al100 | NA | Cut |
| Zaja et al97 | (65) | Cut, Li, Ms, Lu, Gi, O |
| Cutler et al101 | 14/20 (70) | Cut, Ms, Li |
| Mohty et al99 | 10/15 (66) | Cut, Gi, Li, O |
| von Bonin et al103 | 9/13 (69) | Cut, Ms, O |
| Teshima et al102 | 3/7 (43) | Cut, Ms, O |
GVHD indicates graft-versus-host disease; ORR, overall response rate; Cut, skin and mucous membranes; Lu, lung; Ms, musculoskeletal; O, other; Li, liver; NA, not available; and Gi, gastrointestinal.
Overall, according to these studies rituximab was well tolerated. The overall response rate varied between 43% and 80%. The responses usually consisted of partial responses; complete responses were only rarely observed. However, interpretation of the results is complicated by the fact that the patient populations were heterogeneous and response criteria were often not well defined. Chronic GVHD of the skin and musculoskeletal system seems to respond especially well to B-cell depletion, whereas chronic GVHD of the visceral organs seems to respond poorly. Nonetheless, responses of chronic GVHD of the gastrointestinal tract,96,97,99 liver,96-99,101 and lungs97 have been documented occasionally. Several rare manifestations of chronic GVHD such as immune-mediated thrombocytopenia and pure red cell aplasia have also been reported to respond to rituximab treatment.94,104 Addition of rituximab to prednisone may enable successful steroid tapering. B-cell depletion may therefore be a promising approach for the treatment of chronic GVHD, particularly steroid-refractory skin disease. The finding that treatment with rituximab starting 2 months after nonmyeloablative conditioning reduced alloreactive B-cell responses and was associated with a low incidence of chronic GVHD indicates that B-cell depletion could furthermore serve as a GVHD prophylaxis after allogeneic stem cell transplantation.
B cells and the graft-versus-tumor effect
The graft-versus-tumor effect describes the reaction of allogeneic immune cells against malignant cells of the recipient of an allogeneic stem cell transplant. It appears to be responsible in large part for the curative potential of allogeneic stem cell transplantation in malignant hematopoietic diseases. Experimental data addressing the involvement of B cells in the graft-versus-tumor (GVT) effect are scant. A number of studies found an association of antibody responses against tumor-associated antigens with response to donor lymphocyte infusions (DLIs). Bellucci et al reported that the prophylactic administration of donor lymphocyte infusions resulted in a marked polyclonal proliferation of B lymphocytes.105 In a subsequent study the researchers additionally demonstrated that antibody responses to myeloma-associated antigens were associated with complete responses to DLI.106 Furthermore, the antibody response correlated with the time of best response. A similar association of occurrence of a humoral immune response to leukemia with response to DLI was seen in patients with chronic myelogenous leukemia.107 To the same end, alloantibodies to H-Y minor histocompatibility antigens are associated with disease remission.82 Of note, as mentioned before, the presence of H-Y antibodies also confers an increased risk of GVHD. Despite the presumed effectiveness of a conditioning regimen that includes rituximab in preventing acute GVHD, Khouri et al found no evidence of a compromised graft-versus-lymphoma effect.56 These results argue against a role of recipient B cells or donor B cells that are contained in the stem cell graft in the GVT effects. This does not exclude, however, that donor B cells generated de novo might contribute to GVT. If and how tumor antigen–specific B cells facilitate the GVT reaction remain open questions.
B cell–targeted therapies
Several alternative monoclonal anti-CD20 antibodies such as the humanized antibodies ocrelizumab and GA101 as well as the fully human antibody ofatumumab are currently under development. Whether these antibodies provide any advantage over rituximab remains to be shown. Epratuzumab, a novel monoclonal antibody that targets the B-cell antigen CD22, might also prove useful in GVHD. It depletes B cells only incompletely but possesses additional immunomodulatory effects. B cell–tropic factors, such as BAFF, are another promising target for the modification of GVHD in combination with conventional immunosuppressants or rituximab. A number of agents targeting these molecules are currently in preclinical and clinical development. Belimumab is a fully humanized monoclonal anti-BAFF antibody. Its safety and tolerability have been demonstrated in early trials in rheumatoid arthritis and SLE. A newly developed anti-BR3 antibody that combines direct B-cell depletion with blockade of survival signals through the BAFF/BR3-axis is currently in preclinical development. As discussed in the section on chronic GVHD, high BAFF to B-cell ratios correlate with the development of chronic GVHD.89 Because survival signals delivered by BAFF protect autoreactive and alloreactive B cells from in vivo depletion with rituximab and possibly favor their activation and expansion during the subsequent recovery phase, sequential or combined therapy consisting of rituximab and a BAFF-targeting agent might show synergistic effects leading to enhanced efficacy.
Even though they do not specifically target B lymphocytes many standard and experimental therapies for GVHD, including glucocorticoids, mycophenolate mofetil, extracorporeal photopheresis (ECP), and antithymocyte globulin also affect B cells, which might explain part of the beneficial effects of these agents. ECP has been successfully used to treat acute and chronic GVHD with substantial response rates. Exposure of mice to ultraviolet irradiation leads to activation of B lymphocytes with corresponding increase in MHC class II expression but failure to increase the costimulatory molecules CD80 and CD86. These B cells exhibit a strong immunoregulatory activity as was demonstrated by their ability to inhibit the induction of a T-helper 1 (Th1) immune response when coinjected with activated DCs.108 A retrospective analysis of 49 patients treated with ECP discovered that the relative distribution of B-cell subsets could serve as a predictive biomarker for response to ECP.109 In this study, patients who did not respond to ECP had a higher ratio of immature CD19+ CD21− B cells relative to CD19+ CD27+ memory B cells before initiation of therapy. Antithymocyte globulin (ATG), a mixture of purified polyclonal rabbit or horse immunoglobulins against thymocytes, depletes T cells and when given as part of the conditioning before transplantation reduces the incidence of acute GVHD. Besides antibodies against T lymphocytes, ATG contains antibodies reactive with several B cell–specific antigens such as CD19 and CD20 and the plasma cell marker CD138 that can induce B-cell and plasma cell apoptosis.110,111 Surprisingly, a recent study in mice showed that inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, also known as statins, can inhibit acute GVHD.112 Small studies in humans seem to confirm the potential of statins as a GVHD-modifying drug in acute113 and chronic114 GVHD. Statins have pleiotropic immunomodulatory effects on the immune system and inhibit T cells. Interestingly, the immunostimulatory capacity of B cells is exquisitely sensitive to inhibition by statins and requires much lower concentrations of statins than are required for the inhibition of T cells.115
Conclusions
In summary, B cells represent a promising target for the prevention and treatment of GVHD. Although the studies of B-cell depletion in GVHD have shown clinical effectiveness especially in chronic GVHD, the mechanisms underlying the effect are not entirely clear. Therapeutic targeting of B cells with rituximab seems to be a promising adjunct to conventional treatment modalities for GVHD. Formal proof in the form of a prospective, randomized clinical trial with long-term follow-up will still be required, however. One of the major tasks of future research on the role of B cells in GVHD will be to identify better markers for the pathogenic and protective B-cell subsets and to dissect the relative contribution of antibody production, antigen-presentation, and cytokine production by these B cells to the pathogenesis of GVHD. A more detailed mechanistic understanding of the multifaceted role of B cells in GVHD offers the prospect of maximizing the success of B cell–targeted therapy of GVHD by enabling a more selective manipulation of specific B-cell subsets or B cell–specific pathways. The array of B cell–targeting agents that are currently being developed for the treatment of autoimmune disease will ensure that these insights can be quickly translated into new treatments for GVHD.
Acknowledgments
We thank Eisei Kondo and Tanja Liebig for helpful comments and suggestions.
Authorship
Contribution: A.S.-V., M.J.H., R.F.S., and M.S.v.B.-B. cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael S. von Bergwelt-Baildon, Max Eder Junior Research Group and Stem Cell Transplantation Program, Department I of Internal Medicine, University Hospital Cologne, Joseph Stelzmann 9, Cologne, Germany 50937; e-mail: michael.bergwelt@uk-koeln.de.