Abstract
Platelet glycoprotein Ibα (GpIbα) interactions with von Willebrand factor (VWF) are a critical early event in platelet adhesion, which contributes to hemostasis and thrombosis. Here we report the structure of a complex between GpIbα and a potent peptide inhibitor. The cyclic peptide (CTERMALHNLC) was isolated from a cysteine-constrained phage display library, and in the complex this forms one and a half turns of an amphipathic α-helix, the curvature of which facilitates contacts with the curved concave face of the GpIbα leucine-rich repeats. The peptide has only limited overlap with the VWF binding site. It effectively inhibits by stabilizing an alternative α-helical conformation of a regulatory loop that forms an extended β-hairpin upon VWF binding. The structure defines a previously unrecognized binding site within GpIbα and represents a clear strategy for developing antiplatelet agents targeting the GpIbα-VWF interaction allosterically.
Introduction
The interaction between platelet glycoprotein Ibα (GpIbα) and von Willebrand factor (VWF) bound to damaged subendothelium represents the first step in platelet adhesion and is essential for normal hemostasis and vascular repair.1 The interaction is mediated between the N-terminal ligand-binding domain of GpIbα and the VWF-A1 domain and is markedly enhanced as hydrodynamic shear increases, due to conformational activation of VWF or GpIbα or both.2,3 In pathological situations, such as stroke or myocardial infarction, vascular damage and enhanced shear rates occurring in stenotic arteries can cause inappropriate activation of GpIbα-VWF binding contributing to thrombus formation.4 Data using monoclonal antibodies directed at GpIbα have shown promise in targeting the GpIbα-VWF interaction as an antithrombotic therapy with the potential for reduced side effects of bleeding compared with currently available anticoagulants.5 Blocking the GpIbα-VWF interaction is challenging for a small molecule due to the large 2600-Å protein-protein interaction formed in the complex as defined by the GpIbα–VWF-A1 complex crystal structure.6 The OS1 inhibitor is an 11meric peptide that was isolated from a cysteine-constrained phage display library and optimized such that it is capable of selectively disrupting the GpIbα–VWF interaction with a subnanomolar potency.7 To resolve how a small peptide can be such an effective inhibitor of a large protein-protein interaction, we determined the GpIbα-OS1 peptide complex crystal structure.
Methods
Purified protein corresponding to a double deglycosylation mutant of GpIbα residues 1 to 265 (N21Q, N159Q) was prepared as previously described8 (supplemental Methods, available on the Blood website; see the Supplemental Materials link at the top of the online article). The OS1 peptide was synthesized and high-performance liquid chromatography purified by Auspep Ltd. Crystals were grown in sitting drop using 1.9M ammonium sulfate, 80mM lithium sulfate, 100mM N-cyclohexyl-3-aminopropanesulfonic acid buffer, pH 8.2, and a 4-mg/mL protein concentration. Data on a single GpIbα-OS1 crystal were collected on the European Synchrotron Radiation Facility (ESRF) ID23-2 beamline to 1.8-Å resolution. Spacegroup P3121 was determined with unit cell dimensions a = b = 58.8 Å; c = 163.0 Å; α = β = 90; and γ = 120, and data were reduced and processed using the CCP4 software suite9 (MOSFLM). The structure was solved by molecular replacement using Phaser10 with the crystal structure of the N-terminal domain of GpIbα (1GWB)8 as a search model. High-quality 2mFo-DFc and mFo-DFc electron density maps resulted enabling direct location of the bound cyclic peptide (supplemental Video 1). Manual rebuilding was performed using the program Coot11 and refinement using Refmac.12 The peptide was modeled accounting for all 11 residues of the sequence CTERMALHNLC. The final model Rfactor was 22.5% and an Rfree of 28.0% was obtained (supplemental Table 1, supplemental Figure 1).
Results and discussion
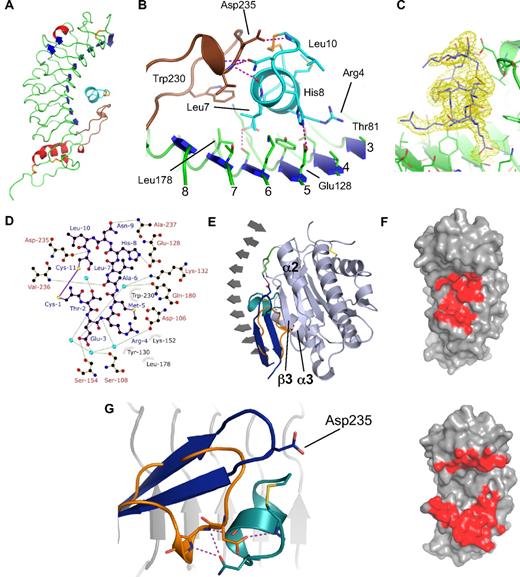
The peptide binds to GpIbα at a site located between the extended regulatory (R) loop and the concave surface of the leucine-rich repeats (LRRs; numbered 1-8; Figure 1A). The peptide adopts one and a half turns of an α-helix and its amphipathic nature presents a hydrophobic face (Ala6, Leu7, and disulphide) to contact the underside of the R-loop and side chains from LRR6 and LRR7, and a hydrophilic face presents charged side chains (Glu3, Arg4, and His8) to contact side chains from LRR3 to LRR7 (Figure 1B). Three subsites are involved in the interaction (S1-S3): S1 and S2 are contacts with the LRRs and S3 is a contact with the R-loop. S1 involves His8 forming a salt bridge with Glu128 and the aliphatic side chain of Arg4 extends over Tyr130 and Val104 with the guanidinium group resting against Thr81 of LRR3. The S2 subsite involves a buried charge as the Glu3 side chain contacts Ser154 and is further buried by hydrophobic contacts with Lys132, Leu178, and Trp230 with the Glu3 carboxylate stabilized by 2 water molecules. Also at this subsite, Leu7 is buried in an extensive hydrophobic pocket composed of Tyr130, Lys152, Thr176, and Leu178. S3 involves interactions with the main chain, and the Asn9 side chain forms 2 hydrogen bonds to the main chain nitrogen atoms of Val236 and Lys237. The main chain nitrogen of Cys11 forms a hydrogen bond with the carboxylate of Asp235 from the R-loop and Ala6 forms the only main chain to main chain interaction through a carbonyl hydrogen bond to the Val236 nitrogen. The Cys1-Cys11 peptide disulphide packs against the main chain peptide bonds from Gly233 and Val234 at the tip of the R-loop. Outside of the subsites, Thr2 does not interact with GpIbα but the side chain oxygen forms an intramolecular α-helix capping hydrogen bond to the Met5 main chain nitrogen.
GpIbα-OS1 peptide complex crystal structure. (A) Ribbon representation of the complex with the regulatory (R) loop colored brown and the peptide colored cyan. Cysteine residues involved in disulphide bonds are highlighted in yellow. (B) Stick representations of GpIbα-OS1 interactions with hydrogen bonds colored as purple dotted lines. Peptide colored in cyan, GpIbα, colored in green with β-sheets highlighted as blue arrows. (C) A 1.8-Å electron density map is shown (yellow) with difference mFo-DFc co-efficients and contoured at 1 root mean square calculated with the OS1 peptide model omitted (program REFMAC). (D) Ligplot figure showing van der Waals and hydrogen bonds formed between GpIbα and OS1. Peptide depicted in purple, polar contacts printed in green. Side chains making hydrophobic interactions are shown as dashed circles. (E) Ribbon diagram of Gplbα (grey) showing only β-strands with OS1 (cyan) superposed onto the Gplbα-VWF-A1 complex with VWF-A1 illustrarted (light blue). The GpIbα R-loop is colored blue and orange for the VWF-A1 and OS1 complexes, respectively, with the Asp235-Lys572 salt bridge highlighted. (F) Binding footprints on the GpIbα surface shown in red for OS1 peptide (top) and VWF-A1 (bottom). (G) The R-loop conformational change from (E) is shown with Asp235 switching from forming a salt bridge to VWF-A1 to coordinating a main chain nitrogen of OS1 peptide. An α-helical form of the R-loop is stabilized by Asn9 from OS1 forming two helix capping hydrogen bonds.
GpIbα-OS1 peptide complex crystal structure. (A) Ribbon representation of the complex with the regulatory (R) loop colored brown and the peptide colored cyan. Cysteine residues involved in disulphide bonds are highlighted in yellow. (B) Stick representations of GpIbα-OS1 interactions with hydrogen bonds colored as purple dotted lines. Peptide colored in cyan, GpIbα, colored in green with β-sheets highlighted as blue arrows. (C) A 1.8-Å electron density map is shown (yellow) with difference mFo-DFc co-efficients and contoured at 1 root mean square calculated with the OS1 peptide model omitted (program REFMAC). (D) Ligplot figure showing van der Waals and hydrogen bonds formed between GpIbα and OS1. Peptide depicted in purple, polar contacts printed in green. Side chains making hydrophobic interactions are shown as dashed circles. (E) Ribbon diagram of Gplbα (grey) showing only β-strands with OS1 (cyan) superposed onto the Gplbα-VWF-A1 complex with VWF-A1 illustrarted (light blue). The GpIbα R-loop is colored blue and orange for the VWF-A1 and OS1 complexes, respectively, with the Asp235-Lys572 salt bridge highlighted. (F) Binding footprints on the GpIbα surface shown in red for OS1 peptide (top) and VWF-A1 (bottom). (G) The R-loop conformational change from (E) is shown with Asp235 switching from forming a salt bridge to VWF-A1 to coordinating a main chain nitrogen of OS1 peptide. An α-helical form of the R-loop is stabilized by Asn9 from OS1 forming two helix capping hydrogen bonds.
The Met5 side chain forms no direct contacts with GpIbα but rests comfortably against the disulphide with the side chain sulfur atom packing between the Thr2 and Leu10 side chains (Figure 1B). In optimizing the GpIbα-binding sequence in OS1, Benard et al7 found a greater degree of diversity at position 5 than other positions, and substitution of Trp from the original peptide to Met (OS1) or Asp (OS2) had a 40- and 2-fold higher dissociation constant for OS1 and OS2, respectively. The GpIbα-OS1 structure suggests the difference in affinity is not due to a direct interaction with GpIbα but rather to improved peptide stability.
A superposition of the GpIbα-OS1 structure with the GpIbα–VWF-A1 complex shows a steric overlap between the peptide and the VWF-A1 domain helix α3 and the β3α2 loop (Figure 1E). OS1 is not a mimetic of the VWF-A1 interaction and the common contacts are limited to residues 236 and 237 in the R-loop with the peptide burying a total area of 500 Å2, which is less than 20% of the surface area buried by VWF-A1 (Figure 1F). The R-loop in the GpIbα–VWF-A1 complex forms a highly ordered extended β-hairpin that is stabilized by the presence of an activating platelet-type von Willebrand disease mutation of residue Met239 to Val that was used in determining the crystal structure.6 The extended R-loop structure is critical to form main chain hydrogen bonds to the central β-sheet of VWF-A1 and residue Asp235 at the tip forms a salt bridge to Lys572 from the VWF-A1 β3α2 loop. In the wild-type uncomplexed GpIbα, the R-loop is poorly ordered, with no electron density observed at the tip for residues 234 to 238.6,8 By contrast, the GpIbα-OS1 structure has a highly ordered density for all residues in the R-loop and a folded back structure exists where Asp235 lies in a 310 helix that is pinned back by a direct helix capping interaction from the peptide Asn9 side chain (Figure 1G). This indicates the peptide has an allosteric component to its inhibition mode, stabilizing a conformation of the R-loop that is incapable of forming key interactions with VWF-A1 (supplemental Video 2).
Platelets are major contributors to arterial thrombosis, and antiplatelet therapy has an established clinical benefit in the treatment and prevention of cardiovascular events. Antiplatelet agents such as integrin αIIbβ3 (glycoprotein IIb/IIIa) inhibitors, aspirin, and clopidogrel have proven efficacy but cardiovascular disease remains an important cause of mortality that has prompted the search for novel drugs against platelet-dependent thrombosis.13 Ligand mimetic peptide complex crystal structures for the platelet receptors integrin αIIbβ3 with RGD14 and α2β1 with a collagen peptide15 have been described, and the former are currently in therapeutic use for treatment of thromboembolic disorders. Our data provide a new scaffold for designing antiplatelet agents targeting the GpIbα–VWF-A1 interaction using small molecules or α-helical peptide derivatives of the OS1 sequence that are capable of occupying the GpIbα allosteric site defined in this complex structure.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the European Synchrotron Radiation Facility for synchrotron facilities. We thank Professor Shaun Jackson for helpful advice on the paper.
This work was supported by the National Health and Medical Research Council of Australia and Monash University (R.K.A.), and by Wellcome Trust grant 061850 (J.E.).
Authorship
Contribution: P.A.M., R.K.A., and J.E. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonas Emsley, Centre for Biomolecular Sciences, RM A10 University Park, Nottingham, NG7 2RD, United Kingdom; e-mail: jonas.emsley@nottingham.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal