Abstract
The molecular mechanisms that regulate the balance between proliferation and differentiation of precursors at the onset of hematopoiesis specification are poorly understood. By using a global gene expression profiling approach during the course of embryonic stem cell differentiation, we identified Sox7 as a potential candidate gene involved in the regulation of blood lineage formation from the mesoderm germ layer. In the present study, we show that Sox7 is transiently expressed in mesodermal precursors as they undergo specification to the hematopoietic program. Sox7 knockdown in vitro significantly decreases the formation of both primitive erythroid and definitive hematopoietic progenitors as well as endothelial progenitors. In contrast, Sox7-sustained expression in the earliest committed hematopoietic precursors promotes the maintenance of their multipotent and self-renewing status. Removal of this differentiation block driven by Sox7-enforced expression leads to the efficient differentiation of hematopoietic progenitors to all erythroid and myeloid lineages. This study identifies Sox7 as a novel and important player in the molecular regulation of the first committed blood precursors. Furthermore, our data demonstrate that the mere sustained expression of Sox7 is sufficient to completely alter the balance between proliferation and differentiation at the onset of hematopoiesis.
Introduction
During embryogenesis, the hematopoietic system develops from mesodermal precursors at specific time and locations. The first mesodermal precursors harboring hematopoietic potential, the hemangioblasts, express the vascular endothelial growth factor receptor 2 (Flk1) and initially were characterized in vitro upon the differentiation of embryonic stem (ES) cells.1,2 Subsequently, these precursors have been identified in vivo in mouse embryos soon after gastrulation3 and in zebrafish.4 Hemangioblast precursors are not fully committed to the blood program because they also give rise to endothelium and smooth muscle.1,5 The emergence of fully restricted hematopoietic precursors, marked by αIIb-integrin (CD41) expression,6-8 occurs soon after the migration of the first hemangioblast to the extraembryonic region.9 These first blood precursors have a restricted potential and give rise only to primitive erythrocyte or macrophage colonies. Definitive precursors, including all myeloid lineages and definitive erythrocytes, are detected at the early somites stage, first in the yolk sac at E8.25, then in the embryo proper at E9.0.
The molecular mechanisms that regulate hematopoiesis specification during embryonic development have been investigated in vivo but also in vitro by use of the differentiation of ES cells as a model system.10 The transcription factors Scl and Runx1 play a central role in the specification of hematopoietic precursors; deficiency in either one of these genes leads to early embryonic lethality. Scl−/− embryos lack all hematopoietic lineages,11 and hemangioblasts derived from Scl−/− ES cells are unable to generate hematopoietic precursors.12,13 Runx1 deficiency results in the absence of definitive hematopoiesis, sparing only primitive erythrocytes.14,15 In vitro experiments using Runx1−/− ES cells have shown a profound defect in the development of hemangioblast precursors.16 Other factors such as Fli1, Gata1, or Gata2 also were shown to be part of the molecular network regulating the specification of hematopoietic precursors.17-20 However, very little is known about the mechanisms that regulate the expansion and homeostasis of these precursors at the onset of specification. Several transcription factors such as HoxB4 or Cdx4 have been shown to enhance the number of hematopoietic progenitors, but their specific function and the subpopulations that they affect remain poorly characterized.21,22
Most members of the Sox family of transcription factors have been implicated in developmental processes ranging from chondrogenesis for Sox9 to sex determination for Sry, the founding member of this family containing 20 genes in mammals.23 Sox genes belong to the high mobility group superfamily and are highly conserved throughout evolution. They are subdivided into 9 groups according to their respective degree of homology in the high mobility group box. A remarkable feature of many Sox factors is their implication in the maintenance of self-renewal and multipotency of stem cells or tissue progenitors, ie, Sox2 for ES cells24 ; Sox10 for neural crest stem cells25 ; Sox1, 2, and 3 for neural progenitors26,27 ; or Sox9 for pancreatic progenitors.28 Another hallmark of the Sox genes is their level of functional redundancy within subgroups in essential developmental processes such as chondrogenesis that can be controlled by either Sox5 or Sox6.29 Sox7 belongs to the F subgroup, which is composed of 3 members, Sox7, 17, and 18. Sox7 and 17 are important for primitive and definitive endoderm specification, respectively.30,31 Sox7 and Sox18 are involved in cardiogenesis and angiogenesis, playing redundant role in both of these processes.32-35 Sox17, the only member of this subgroup implicated in hematopoiesis, regulates the maintenance of fetal and neonatal hematopoietic stem cells.36 To date no role has been assigned for Sox7 in hematopoiesis.
In the present study, we identify Sox7 as a gene specifically expressed in Flk1+ mesodermal precursors. We investigate its potential role in hematopoiesis using the in vitro differentiation of ES cells as a model system, as well as embryos at the onset of hematopoietic development. We show that Sox7 expression is up-regulated in Flk1+ mesodermal precursors then progressively down-regulated as differentiation progresses toward mature blood precursors for primitive and definitive lineages. Knockdown of Sox7 expression using shRNA results in a significant decrease in the formation of hematopoietic progenitors. By using a Tet-on doxycycline-inducible expression system, we show that enforced expression of Sox7 in hematopoietic precursors prevents their differentiation to mature primitive and definitive lineages while maintaining their self-renewal potential. Our study identifies Sox7 as a novel player in the molecular regulation of the first committed blood precursors.
Methods
ES cell growth and differentiation
ES cell growth and embryoid body (EB) differentiation were performed as previously described.37 The generation of hemangioblast-derived blast cell colonies and hematopoietic precursors in clonogenic assays were performed in semisolid hemangioblast and hematopoietic mix, respectively. For liquid culture, an equivalent volume of Iscove modified Dulbecco medium replaced the methylcellulose. Definitive colonies represent all definitive colonies, including macrophages colonies, macrophages/erythrocytes colonies, granulomacrophage colonies, and mixed granulomacrophage-erythroid colonies. Scoring data for all colonies are shown from a representative experiment as the mean number of colonies from 3 dishes. Bars, where visible, represent standard error of the mean.
Gene expression analysis
Total RNA was extracted with an RNeasy kit, treated with RNAse-free DNase (QIAGEN), and reverse-transcribed into cDNA with random hexamer by use of an Omniscript RT kit (QIAGEN). Polymerase chain reactions (PCRs) were performed by the use of Go Taq (Bioline) and 0.4μM of each gene-specific oligonucleotides primer; sequences are available upon request. Real-time PCRs were performed on an ABI 7900 system (Applied Biosystems) by use of the Exiqon universal probe library and primer designer (Roche). All expression data were calculated relative to β-actin as 2−Δct. Data are presented as ΔCt values from triplicates normalized to β-actin.
Flow cytometry
EBs or embryos were trypsinized (TryplE; Gibco) for 3 minutes, and the single-cell suspension was analyzed for green fluorescent protein (GFP) expression or further stained. Cells were blocked with FcRγII/III antibody (24G2 supernatant) before staining with various combinations of Flk1-bio, CD41-PE, CD34-bio, Ter119-PE, cKit-APC, CD71-bio, (PharMingen), CD11b-PE, Tie2-bio, and VE-cadherin-647 (eBioscience) followed by strep-Cy7 or strep-Cy5 (PharMingen). Cells were analyzed on a FacsCalibur flow cytometer or sorted on a FacsAria (BD Biosciences). 5-Bromo-2-deoxyuridine (BrdU) incorporation (BrdU Flow kit, BD Pharmingen) and annexin V staining (PE Annexin V apoptosis detection kit I; BD Pharmingen) were performed according to the manufacturer's instructions.
Bacterial artificial chromosome transgene cloning
Bacterial artificial chromosome (ID: 250F17) was purchased from mouse bMQ bacterial artificial chromosome (BAC) library (Wellcome Trust Sanger Institute). All recombineering steps were performed as previously described.38 In brief, we replaced Sox7 exon 1 at the ATG start of transcription with a β-globin intron (IVS) eGFP cassette. Kanamycin in bacterial cells and neomycin resistance in mammalian cells were used as selection markers after electroporation. Positive clones were confirmed by PCR amplification and restriction enzyme DNA digestion.
Transgenic mice and embryo generation
Timed matings were set up between transgenic male (Sox7-GFP+ or iSox7+rtTA+) and wild-type C57Bl/6 female mice. The morning of vaginal plug detection was embryonic day (E) 0.5. Gastrulating embryos were staged by morphologic landmarks.39 All animal work was performed under regulations governed by the Home Office Legislation under the Animal Scientific Procedures Act of 1986.
ShRNA and lentivirus production and transduction
Nonsilencing and Sox7 shRNA were purchased from Open Biosystems and expressed in a modified version of the pGIPZ with an Ef1 promoter replacing the CMV promoter. VSVg pseudotyped lentiviral vectors were produced by use of the third-generation self-inactivating vector system as described.40 In brief, viral supernatant was harvested from HEK293T cells at days 2, 3, and 4 after calcium phosphate transfection. Supernatant was passed through a 0.45-μm filter (Millipore), concentrated by ultra centrifugation (2 hours, 20 000g, 4°C Beckman Coulter optima L-90K ultracentrifuge), resuspended in phosphate-buffered saline, aliquoted, and stored at −80°C. Viral titers were expressed as HeLa-cell–transducing units per milliliter (TU/mL) as determined by flow cytometry for GFP 72 hours after transduction. Sorted mesodermal precursors were transduced at a multiplicity of infection of 20 in EB media and plated in ultralow attachment dishes (Corning). After 2 days, aggregates were analyzed as previously described.41
Statistical analysis
Each of the experiments was repeated at least 3 times. Differences between the control and experimental conditions were compared by use of a t test. Statistical significance was accepted when P was less than .05.
Results
Sox7 is transiently expressed at the onset of hematopoietic specification
The use of ES cells with GFP knocked in the brachyury locus (referred to as Bry-GFP) allows, upon in vitro differentiation, to separate subpopulations representing different stages of development based on their levels of GFP and Flk1 expression. Bry-GFP−Flk1− cells have epiblast-like characteristics,42 and Bry-GFP+Flk1− cells are mesodermal precursors, which give rise upon further differentiation to Flk1+ cells containing hemangioblast precursors.41 By using a global gene expression profiling approach to define novel genes expressed at the onset of blood specification, we identified Sox7 as a gene specifically up-regulated in the Bry-GFP+Flk1+ population.
Reverse transcription (RT)-PCR analysis confirmed the specific expression of Sox7 in the Flk1+ subpopulation enriched in hemangioblasts (Figure 1A) along with genes indicative of hematopoietic specification such as Runx1 and Scl, known to be critical at the onset of blood development.11,15 When cultured in the presence of vascular endothelial growth factor, GFP-Bry+Flk1+ hemangioblast precursors generate blast colonies containing both primitive and definitive hematopoietic progenitors.1 To define the dynamic of Sox7 expression along the progressive specification of hemangioblasts to fully committed hematopoietic precursors, we analyzed its expression level during the 4-day time course of blast colony formation. Both Scl and Runx1 expression remained high and stable during hemangioblast-derived blast colony formation (Figure 1B). The expression of βH1-globin, indicative of primitive erythropoiesis, was up-regulated by day 2, whereas PU.1 expression, marking myeloid maturation, was up-regulated by day 3. Interestingly, Sox7 expression was first up-regulated on day 1 (4-fold relative to its expression in Flk1+ cells) but then down-regulated from day 2 onward (Figure 1B-C).
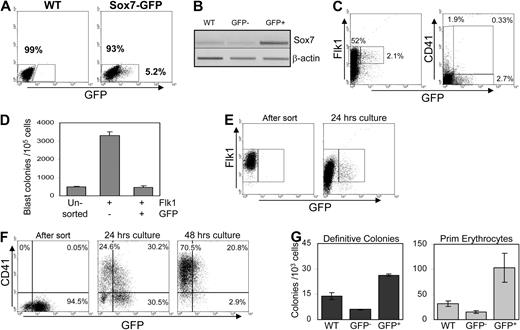
Sox7 at the onset of hematopoiesis specification. (A) Gene expression analysis by RT-PCR performed on day 3 EB subpopulations sorted for their respective expression of Bry-GFP and Flk1. (B) Expression analysis by RT-PCR on hemangioblast-derived blast colonies grown from sorted Flk1+ plated in semisolid clonogenic assay and harvested at the indicated time points. (C) Relative quantification of Sox7 expression by real-time PCR in the indicated population of Bry-GFP and Flk1 sorted at day 3 EBs and days 1 to 4 hemangioblast-derived blast colonies. Sorted Bry-GFP+Flk1−, mesodermal precursors were transduced with the indicated shRNA-expressing lentivirus, cultured for 2 days, and then tested for hematopoietic progenitors (D) and for endothelial potential (E) in clonogenic replating assay (n = 5). (F) Total cell recovery from mesodermal precursors transduced with the indicated virus after 2 days in culture. (G) Flow cytometric analysis of annexin V and 7-aminoactinomycin D staining on mesodermal precursors transduced by the indicated virus after 1 day of culture. All data are representative of at least 3 independent experiments. Error bars, where visible, represent SEM. *P < .01, **P < .05 compared with control shRNA.
Sox7 at the onset of hematopoiesis specification. (A) Gene expression analysis by RT-PCR performed on day 3 EB subpopulations sorted for their respective expression of Bry-GFP and Flk1. (B) Expression analysis by RT-PCR on hemangioblast-derived blast colonies grown from sorted Flk1+ plated in semisolid clonogenic assay and harvested at the indicated time points. (C) Relative quantification of Sox7 expression by real-time PCR in the indicated population of Bry-GFP and Flk1 sorted at day 3 EBs and days 1 to 4 hemangioblast-derived blast colonies. Sorted Bry-GFP+Flk1−, mesodermal precursors were transduced with the indicated shRNA-expressing lentivirus, cultured for 2 days, and then tested for hematopoietic progenitors (D) and for endothelial potential (E) in clonogenic replating assay (n = 5). (F) Total cell recovery from mesodermal precursors transduced with the indicated virus after 2 days in culture. (G) Flow cytometric analysis of annexin V and 7-aminoactinomycin D staining on mesodermal precursors transduced by the indicated virus after 1 day of culture. All data are representative of at least 3 independent experiments. Error bars, where visible, represent SEM. *P < .01, **P < .05 compared with control shRNA.
To examine the requirement for Sox7 during hematopoietic precursor formation, we made use of the shRNA technology to knockdown its expression in mesodermal precursors as they formed blood precursors. Bry-GFP+Flk1− cells, which are the direct mesodermal precursors of Bry–GFP+Flk1+ cells as previously shown,41 were isolated from day 2.5 EBs, transduced with lentivirus expressing either control nonsilencing shRNA or 1 of the 3 Sox7 shRNA that efficiently down-regulated Sox7 mRNA level (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). After 2 days in culture, the transduced cells were tested for hematopoietic potential in clonogenic replating assay (Figure 1D). Although the control shRNA had a slight nonsignificant impact on the number of hematopoietic precursors formed, decreasing Sox7 expression by the use of 3 independent shRNA constructs significantly reduced the total number of both primitive and definitive colonies generated. Notably, we observed a correlation between the level of Sox7 knockdown and the decrease in hematopoietic progenitor formation (Figure 1D and supplemental Figure 1C). Both Sox7a and Sox7c shRNA efficiently down-regulated Sox7 expression and resulted in low blood progenitor formation. In contrast, Sox7b shRNA was less efficient at down-regulating Sox7 expression and led to a less-drastic reduction of hematopoietic progenitors. The potential to generate endothelial colonies was also assayed for the Sox7 knockdown mesodermal precursors in clonogenic replating (Figure 1E). Similar to the hematopoietic potential, we observed a strong reduction in the ability to form endothelial colonies when Sox7 expression was knockdown. To investigate the underlying basis for the important decrease in both potentials, we analyzed the survival and proliferative status of the mesodermal precursors transduced with lentivirus-expressing shRNA. After 2 days of culture, the number of cells recovered from Sox7 shRNA cultures was on average 6.4 times lower than from the control shRNA cultures (Figure 1F). Furthermore, we also observed a greater frequency of cells undergoing apoptosis as shown by increased annexin V and 7AAD staining (supplemental Table 1; Figure 1G).
Taken together, these data indicate that Sox7 expression is transiently up-regulated at the onset of blood specification. The knockdown of Sox7 expression in mesodermal precursors results in a significant decrease in the number of primitive and definitive hematopoietic progenitors as well as endothelial precursors. The increased level of apoptosis observed upon Sox7 knockdown further suggests a requirement for this transcription factor in the survival of both hematopoietic and endothelial precursors during specification.
Sox7 is expressed in the first committed hematopoietic progenitors
To identify the cells that transiently express Sox7, we engineered a BAC transgene containing the complete Sox7 locus with the first exon replaced by a GFP-neomycin cassette (supplemental Figure 2A). Several ES cell lines carrying this transgene (referred to as Sox7-GFP) were tested and gave similar results. GFP− and GFP+ cells were sorted from day 3 EBs to assess their enrichment for endogenous Sox7 expression (Figure 2A). As expected, Sox7 expression effectively segregated to the GFP+ fraction, indicating that the Sox7–GFP transgene adequately paralleled the endogenous pattern of Sox7 expression (Figure 2B). No significant effect was observed in the differentiation of ES cells carrying the Sox7–GFP transgene, as illustrated by the expected frequency of primitive and definitive hematopoietic progenitors (supplemental Figure 2B). At day 3 of differentiation, 2% to 3% of EB cells expressed Sox7–GFP, a fraction of which coexpressed Flk1, whereas very few coexpressed CD41+ (Figure 2C). Interestingly however, Sox7-GFP did not mark hemangioblast precursors because Flk1+Sox7-GFP+ cells were not enriched for these progenitors relative to Flk1+Sox7-GFP− cells (Figure 2D).
Sox7 is expressed at the transition from hemangioblast to CD41 up-regulation. (A) Sox7-GFP expression in day 3 EBs derived from an ES cell line transgenic for the Sox7-GFP BAC Construct. WT indicates wild type. (B) Expression of endogenous Sox7 was assessed by RT-PCR on GFP+ and GFP− cells sorted from day 3 wt and Sox7-GFP EBs. (C) Flow cytometric analysis of day 3 Sox7-GFP EBs for Flk1 and CD41 expression. (D) Cells from day 3 Sox7-GFP EBs were sorted based on Flk1 and GFP expression and replated in clonogenic assay for hemangioblast progenitors. (E) Sorted Flk1+Sox7-GFP− cells were cultured for 24 hours in liquid hemangioblast mix then analyzed for Sox7-GFP+ expression. Flk1 staining after 24 hours represents remaining staining from sort. (F) Sorted Flk1+Sox7–GFP+ cells were cultured for 24 or 48 hours in liquid hemangioblast mix then analyzed for CD41 and Sox7–GFP expression. (G) After 48 hours of culture, wt Flk1+ cells, Flk1+Sox7-GFP−, and Flk1+Sox7-GFP+ cells were tested for the presence of hematopoietic progenitors in semisolid clonogenic replating assay. All data are representative of at least 3 independent experiments. Error bars, where visible, represent SEM.
Sox7 is expressed at the transition from hemangioblast to CD41 up-regulation. (A) Sox7-GFP expression in day 3 EBs derived from an ES cell line transgenic for the Sox7-GFP BAC Construct. WT indicates wild type. (B) Expression of endogenous Sox7 was assessed by RT-PCR on GFP+ and GFP− cells sorted from day 3 wt and Sox7-GFP EBs. (C) Flow cytometric analysis of day 3 Sox7-GFP EBs for Flk1 and CD41 expression. (D) Cells from day 3 Sox7-GFP EBs were sorted based on Flk1 and GFP expression and replated in clonogenic assay for hemangioblast progenitors. (E) Sorted Flk1+Sox7-GFP− cells were cultured for 24 hours in liquid hemangioblast mix then analyzed for Sox7-GFP+ expression. Flk1 staining after 24 hours represents remaining staining from sort. (F) Sorted Flk1+Sox7–GFP+ cells were cultured for 24 or 48 hours in liquid hemangioblast mix then analyzed for CD41 and Sox7–GFP expression. (G) After 48 hours of culture, wt Flk1+ cells, Flk1+Sox7-GFP−, and Flk1+Sox7-GFP+ cells were tested for the presence of hematopoietic progenitors in semisolid clonogenic replating assay. All data are representative of at least 3 independent experiments. Error bars, where visible, represent SEM.
To establish the relationship between the subpopulations expressing Flk1, CD41, and Sox7–GFP, we analyzed via cell sorting and subsequent culture the progression from one subpopulation to the others. When Flk1+Sox7-GFP−-sorted cells were cultured for 24 hours, they gave rise to Sox7–GFP+ cells (Figure 2E), demonstrating that Sox7 expression was up-regulated within a subset of Flk1+ cells. Initially, the Flk1+Sox7–GFP+-sorted cells did not express CD41 (Figure 2F left panel), but when cultured for 24 hours, they gave rise to a large fraction of CD41+ cells expressing lower levels of Sox7-GFP (Figure 2F middle panel). After 48 hours, more than 70% of the cells were CD41+ and had mostly down-regulated Sox7–GFP expression. These data indicate that Sox7 was transiently expressed in Flk1+ cells, giving rise to CD41+ cells. Analysis of the hematopoietic potential of sorted Flk1+Sox7–GFP+ cells cultured for 48 hours revealed the presence of a high frequency of primitive and definitive hematopoietic precursors relative to total wild-type Flk1+ or to the Flk1+Sox7–GFP− subfraction cultured in a similar way (Figure 2G). Altogether, these results indicate that Sox7 is transiently expressed as Flk1+ cells become committed to CD41+ hematopoietic precursors for both primitive and definitive lineages. Thus, Sox7 expression appears to define a narrow window of development between hemangioblast specification and hematopoietic commitment.
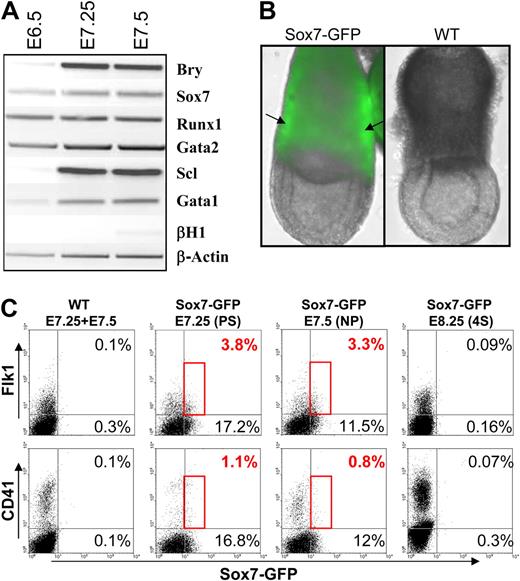
To address the in vivo relevance of this finding, we first confirmed by gene expression analysis that Sox7 was expressed in gastrulating embryos along with other transcription factors implicated in hematopoiesis (Figure 3A). To refine this analysis, we established a transgenic mouse line derived from the Sox7–GFP ES cells. The direct detection of GFP expression in gastrulating embryos revealed the presence of GFP expression in the yolk sac region of Sox7–GFP embryos (Figure 3B). Interestingly, regions of brighter intensity could be observed at locations where mesodermal masses would later give rise to blood islands. Flow cytometric analysis of pooled embryos from either wt or Sox7–GFP litters revealed the presence of a small subpopulation of Flk1+ cells coexpressing Sox7–GFP at late primitive streak (PS) and neural plate (NP) stages of development, whereas this population became undetectable from the 4 somites stage onward (Figure 3C). As soon as Flk1+ cells were detected in embryos, we were able to observe some level of Sox7–GFP expression (data not shown). A smaller fraction of Sox7–GFP+ cells also coexpressed CD41 at these early stages of development (Figure 3C, bottom panels). A large fraction of Sox7–GFP+ cells expressed neither Flk1 nor CD41 and likely represented primitive endoderm that localizes to the yolk sac part of the embryo at these stages of development.31 Altogether, these data support our in vitro results, indicating that Sox7 expression can be detected transiently in gastrulating embryos both in Flk1+ and CD41+ cells.
Sox7 expression in gastrulating embryos. (A) Gene expression analysis performed by RT-PCR on pools of wt embryos at the indicated stage of development. (B) Pictures of E7.5 embryo wild-type (WT) or transgenic for the Sox7-GFP transgene. Arrows indicate the mesodermal masses. Merge picture of GFP and brightfield (×10 magnification). (C) Stage-matched pool of embryos from WT or Sox7-GFP litters stained for Flk1 and CD41 expression. 4S indicates 4 somites. All data are representative of at least 3 independent experiments.
Sox7 expression in gastrulating embryos. (A) Gene expression analysis performed by RT-PCR on pools of wt embryos at the indicated stage of development. (B) Pictures of E7.5 embryo wild-type (WT) or transgenic for the Sox7-GFP transgene. Arrows indicate the mesodermal masses. Merge picture of GFP and brightfield (×10 magnification). (C) Stage-matched pool of embryos from WT or Sox7-GFP litters stained for Flk1 and CD41 expression. 4S indicates 4 somites. All data are representative of at least 3 independent experiments.
Sox7-enforced expression blocks hematopoietic differentiation
To further investigate the role of Sox7 at the onset of hematopoietic specification and define the significance of its down-regulation upon differentiation, we analyzed the effect of Sox7-sustained expression in hematopoietic precursors. By the use of a doxycycline-inducible system previously described,21 we established ES cell clones carrying a Sox7–2A–GFP cDNA (referred to as iSox7). In the presence of doxycycline, iSox7 ES cells were induced to express in bicistronic mode Sox7 and GFP proteins via the efficient internal cleavage of the 2A peptidic sequence,43 allowing us to track Sox7-expressing cells with GFP (supplemental Figure 3A-B). Single-cell suspension of iSox7 day 5 EBs, which contains primitive and definitive hematopoietic precursors, was replated in clonogenic assay with or without doxycycline to assess the outcome of Sox7-enforced expression on the growth of hematopoietic colonies. In the absence of doxycycline, iSox7 cells gave rise to the expected formation of all colony types, whereas in the presence of doxycycline very few primitive or definitive colonies were detected (Figure 4A-B).
Sox7-enforced expression blocks hematopoietic differentiation. (A) Pictures of colonies from day 5 iSox7 EB cells noninduced (−dox) or induced (+dox) with doxycycline at 1 μg/mL in semisolid clonogenic replating assay for hematopoietic progenitors 5 days after replating (×4 magnification). (B) Colony counts from day 5 iSox7 EB cells replated with or without doxycycline (+dox, −dox) in clonogenic assay for hematopoietic progenitors. (C) Representative pictures of blastic colonies obtained in clonogenic replating assay with doxycycline (×40 magnification). (D) Individual colonies grown without or with doxycycline tested for the expression of the indicated genes by real-time PCR. (E) Flow cytometric analysis for the indicated cell surface markers of pooled colonies grown for 5 days in semisolid clonogenic replating for hematopoietic progenitors without or with doxycycline. (F) May-Grünwald Giemsa and O-dianidisine staining of cells derived from sorted hematopoietic precursors grown for 6 days in liquid cultures with or without doxycycline (black arrows indicate primitive erythrocytes; green arrows, macrophages; and red arrows, blast cells). All data are representative of at least 3 independent experiments.
Sox7-enforced expression blocks hematopoietic differentiation. (A) Pictures of colonies from day 5 iSox7 EB cells noninduced (−dox) or induced (+dox) with doxycycline at 1 μg/mL in semisolid clonogenic replating assay for hematopoietic progenitors 5 days after replating (×4 magnification). (B) Colony counts from day 5 iSox7 EB cells replated with or without doxycycline (+dox, −dox) in clonogenic assay for hematopoietic progenitors. (C) Representative pictures of blastic colonies obtained in clonogenic replating assay with doxycycline (×40 magnification). (D) Individual colonies grown without or with doxycycline tested for the expression of the indicated genes by real-time PCR. (E) Flow cytometric analysis for the indicated cell surface markers of pooled colonies grown for 5 days in semisolid clonogenic replating for hematopoietic progenitors without or with doxycycline. (F) May-Grünwald Giemsa and O-dianidisine staining of cells derived from sorted hematopoietic precursors grown for 6 days in liquid cultures with or without doxycycline (black arrows indicate primitive erythrocytes; green arrows, macrophages; and red arrows, blast cells). All data are representative of at least 3 independent experiments.
Quite surprisingly, however, a large number of colonies, immature in appearance, were observed when Sox7 expression was induced. These colonies were uniformly GFP positive (Figure 4C), expressed Scl and Gata1, both indicative of hematopoietic commitment, and Sox7 (Figure 4D) with, on average, an 11-fold greater level than the endogenous expression detected at day 1 of hemangioblast differentiation (supplemental Figure 3C). Similar results were observed with 3 independent iSox7 ES clones (not shown). Analysis of differentiation markers on pooled colonies indicated that without induction, approximately 10% of the cells expressed CD11b or Ter119, marking, respectively, macrophage-granulocyte and erythroid lineages (Figure 4E). In contrast, upon Sox7 induction, most cells were GFP+, but few expressed CD11b or Ter119. To further explore the immunophenotype of iSox7+ colonies, we analyzed levels of CD34 and CD41 expression, both expressed at early stages of differentiation. Sox7-enforced expression resulted in high level of detection of both markers, including subsets expressing variable levels of these 2 markers CD41+CD34− and CD41lowCD34+ (Figure 4E). Noninduced cells expressed much lower level of CD34 and little CD41. In terms of morphology, most noninduced cells had typical macrophage or erythrocyte morphologies. In contrast, Sox7-expressing cells were mostly blastlike in appearance with little evidence of morphologic changes to maturing cells (Figure 4F). To identify nonspecific effects induced either by GFP or doxycycline, we performed similar experiments with ES clones carrying a 2A-GFP inducible construct (referred to as i2A-GFP) and observed no changes in primitive and definitive hematopoietic potential upon doxycycline induction (supplemental Figure 3D-E). Taken together, our data suggest that the sustained expression of Sox7 beyond its normal time frame of expression in hematopoietic precursors prevents their differentiation to more mature cells.
Sox7-enforced expression maintains the proliferative potential of the earliest committed hematopoietic precursors
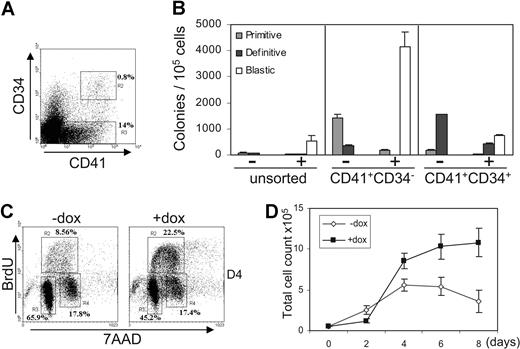
Early hematopoietic precursors can be subdivided according to their relative expression of CD41 and CD34 (Figure 5A). At day 5 of EB differentiation, all precursors are found within the CD41+CD34− and CD41+CD34+ subsets as previously shown.44 To define which subpopulation of precursors was most affected by Sox7-enforced expression, we induced Sox7 in CD41+CD34−- and CD41+CD34+-sorted populations (Figure 5B). The most immature CD41+CD34− precursors appeared to be more susceptible to the differentiation block mediated by Sox7 than the CD41+CD34+ subpopulation. Upon induction, CD41+CD34+ precursors gave rise to fewer blastic colonies and more mature definitive colonies. The number of blastic colonies obtained from CD41+CD34− cells exceeded the total number of both primitive and definitive colonies generated without induction, suggesting that Sox7-sustained expression allowed the survival and proliferation of precursors that would not otherwise form colonies.
Sox7-enforced expression maintains the proliferation of early hematopoietic precursors. (A) CD41 and CD34 staining on cells derived from day 5 iSox7 EBs. (B) Unsorted, CD41+CD34−-, and CD41+CD34+-sorted cells from day 5 iSox7 EBs were tested in semisolid clonogenic replating assay for hematopoietic progenitors with or without doxycycline. (C) Cell-cycle status was assessed after 1 hour BrdU pulse on CD41+CD34− cells culture for 4 days (D4) +dox or −dox. (D) Sorted CD41+CD34− cells were cultured for 8 days + or − doxycycline. Total cell count was determined every other day (n = 5). All data are representative of at least 3 independent experiments. Error bars represent SEM.
Sox7-enforced expression maintains the proliferation of early hematopoietic precursors. (A) CD41 and CD34 staining on cells derived from day 5 iSox7 EBs. (B) Unsorted, CD41+CD34−-, and CD41+CD34+-sorted cells from day 5 iSox7 EBs were tested in semisolid clonogenic replating assay for hematopoietic progenitors with or without doxycycline. (C) Cell-cycle status was assessed after 1 hour BrdU pulse on CD41+CD34− cells culture for 4 days (D4) +dox or −dox. (D) Sorted CD41+CD34− cells were cultured for 8 days + or − doxycycline. Total cell count was determined every other day (n = 5). All data are representative of at least 3 independent experiments. Error bars represent SEM.
By using this enriched subpopulation of precursors giving rise to blastic colonies, we next assessed their proliferation potential. Incorporation of BrdU in sorted cells upon culture revealed that Sox7-enforced expression led to a greater fraction of cells in S phase (Figure 5C). Immuno-fluorescence staining for Ki67 antibody, an indicator of cell proliferation, further substantiated this enhanced level of proliferation (supplemental Figure 4). Furthermore, total cell count during an 8-day time course showed that without induction CD41+CD34− first expanded but then stopped proliferating as cells differentiated to mature lineages. In contrast, Sox7-induced cells, after a 2-day lag-period, expanded steadily without sign of differentiation (Figure 5D and 4F). Altogether, these data suggest that Sox7-enforced expression maintains or enhances the proliferative potential of CD41+CD34− hematopoietic precursors while preventing their differentiation to mature blood lineages.
Release from Sox7-enforced expression leads to the formation of erythroid and myeloid lineages
In the model system used here, Sox7 expression is controlled by addition and removal of doxycycline. Taking advantage of this flexibility, we next determined the biologic potential of iSox7+ cells upon doxycycline removal in media with cytokines supporting the growth of all myeloid and erythroid lineages. Doxycycline removal led to the rapid down-regulation of GFP; within 2 days GFP was no longer detectable (Figure 6A). The appearance of a Ter119+CD71+ erythrocyte population was observed 2 days after the removal of doxycycline and peaked at day 6 with more than 50% of Ter119+CD71+ cells (Figure 6B, top). This progressive differentiation induced by doxycycline withdrawal was also illustrated by the accumulation of CD34+cKit+ mast cells that became predominant by day 8 (Figure 6B, bottom). Cell morphology analysis indicated that cells from iSox7+ blastic colonies cultured without doxycycline gave rise predominantly to erythrocytes after 6 days and mostly mast cells after 10 days, whereas macrophages and megakaryocytes were observed at all stages of the culture (Figure 6C and data not shown).
Sox7 down-regulation leads to erythroid and myeloid differentiation. (A) GFP expression on pooled colonies harvested after 6 days in liquid hematopoietic conditions with doxycycline (red line) compared with GFP expression after doxycycline removal for 1 day (black line) and 2 days (green line). (B) Flow cytometry analysis of iSox7+ cells cultured in liquid hematopoietic conditions for the indicated time without doxycycline. (C) May-Grünwald Giemsa staining at days 6 and 10 of culture after doxycycline removal. (D) After May-Grünwald Giemsa staining, the percentage of erythroid and myeloid cell types was scored from individual iSox7+ colonies grown for 5 days without doxycycline. For each colony, 100 cells were counted. (E) Individual iSox7+ blastic colonies from clonogenic replating with doxycycline were plated in secondary clonogenic assay without doxycycline. All data are representative of at least 3 independent experiments.
Sox7 down-regulation leads to erythroid and myeloid differentiation. (A) GFP expression on pooled colonies harvested after 6 days in liquid hematopoietic conditions with doxycycline (red line) compared with GFP expression after doxycycline removal for 1 day (black line) and 2 days (green line). (B) Flow cytometry analysis of iSox7+ cells cultured in liquid hematopoietic conditions for the indicated time without doxycycline. (C) May-Grünwald Giemsa staining at days 6 and 10 of culture after doxycycline removal. (D) After May-Grünwald Giemsa staining, the percentage of erythroid and myeloid cell types was scored from individual iSox7+ colonies grown for 5 days without doxycycline. For each colony, 100 cells were counted. (E) Individual iSox7+ blastic colonies from clonogenic replating with doxycycline were plated in secondary clonogenic assay without doxycycline. All data are representative of at least 3 independent experiments.
To study at a clonal level the potential of these blastic colonies, individual iSox7+ colonies were picked and expanded without doxycycline. After 6 days in liquid culture, most colonies (> 90%) gave rise to erythrocytes, macrophages, neutrophils, and megakaryocytes (Figure 6D). The clonogenic potential of iSox7+ colonies was next determined in semisolid replating without doxycycline. Most individually picked colonies contained a high frequency of progenitors developing into single-lineage colonies such as primitive erythrocytes or macrophages but also multilineage colonies of granulomacrophages or mixed lineages (Figure 6E). Because hematopoietic and endothelium lineages can be derived from the same precursor, we also assessed the potential of these colonies to generate endothelium. However, in 2 independent assays, iSox7+ cells failed to give rise to endothelial cells. Single-cell suspension derived from iSox7+ colonies did not form endothelial colonies in semisolid assay unlike Flk1+ cells derived from day 4 EB (not shown). Individually picked iSox7+ colonies were equally unable to form tubule-like structures in Matrigel plug unlike endothelium colonies derived from Flk1+ cells (not shown).
Altogether, these data indicate that removing the developmental block initiated by the maintenance of Sox7 expression leads to the formation of erythroid and multiple myeloid lineages. Furthermore, these data suggest that Sox7 expression maintains multipotential hematopoietic precursors that have lost their potential to form endothelium or, alternatively, that Sox7 expression is able to restrict the potential of precursors to the hematopoietic fate only.
Gastrulating embryos contain Sox7-responsive hematopoietic precursors
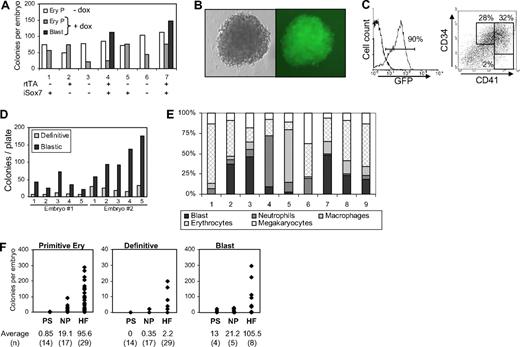
To establish whether similar precursors responsive to Sox7-enforced expression could be identified in vivo during early embryonic development, we derived a transgenic mouse line from iSox7 ES cells. At E7.5 individual embryos obtained from the iSox7 mouse line were plated in clonogenic assay with or without doxycycline. At this stage of development, all embryos contained precursors for primitive erythrocytes colonies but no precursors for definitive colonies without doxycycline (Figure 7A). Interestingly, cells derived from embryos, carrying both the rtTA and iSox7 transgenes, gave rise to blastic colonies in the presence of doxycycline. Gastrulating embryos from the control transgenic mouse line carrying the 2A–GFP construct never formed blastic colonies (not shown). The blastic colonies derived from iSox7 embryos were uniformly positive for GFP and morphologically identical to the colonies derived from the in vitro culture experiments (Figure 7B). These colonies expressed high levels of Sox7, as detected by real-time PCR performed on individual colonies (not shown). Phenotypic analysis of pooled colonies revealed that most cells within these colonies were positive for GFP, with more than 60% coexpressing CD34 and CD41 (Figure 7C). Secondary replating of individual embryo-derived colonies with doxycycline led to the formation of more blastic colonies (Figure 7D) that were further amplified in tertiary replating (not shown). This serial replating capacity demonstrated the high proliferative potential of these embryo-derived cells upon Sox7-enforced expression. The hematopoietic potential of these iSox7+ colonies was next determined upon culture of individual colonies without doxycycline (Figure 7E). Most colonies gave rise to erythrocytes, macrophages, neutrophils, and megakaryocytes, which demonstrated the multipotential nature of these embryo-derived colonies.
Gastrulating embryos contain Sox7-responsive hematopoietic precursors. (A) Cells from E7.5 embryos were tested in clonogenic replating assay for hematopoietic progenitors with or without doxycycline (+dox, −dox). For each embryo, 1/10 of the cells was used for genotyping and the remaining divided equally in −dox and +dox replating conditions. Genotype for rtTA and iSox7 transgenes is shown below the graph. (B) Picture of a blastic colony from replating with doxycycline (×20 magnification). (C) Flow cytometric analysis of pooled colonies grown for 6 days in secondary replating with doxycycline. (D) 5 individual iSox7+ colonies from primary replating of 2 different embryos were replated in secondary clonogenic assay with doxycycline. (E) After May-Grünwald Giemsa staining, erythrocyte and myeloid cells were scored from individual iSox7+ colonies grown for 5 days in liquid hematopoietic conditions without doxycycline. A total of 100 cells were counted per colony. (F) Cells from individual embryos were tested in clonogenic replating assay for hematopoietic progenitors with or without doxycycline. For each embryo, 1/10 of the cells was used for genotyping and the remaining equally divided in −dox and +dox replating conditions. HF indicates headfold. The average numbers for each colony type are presented below the graphs; (n) represents the number of embryo analyzed at each stage of development. For blastic colonies, only embryos positive for rtTA and isox7 transgenes are depicted. All data are representative of at least 3 independent experiments.
Gastrulating embryos contain Sox7-responsive hematopoietic precursors. (A) Cells from E7.5 embryos were tested in clonogenic replating assay for hematopoietic progenitors with or without doxycycline (+dox, −dox). For each embryo, 1/10 of the cells was used for genotyping and the remaining divided equally in −dox and +dox replating conditions. Genotype for rtTA and iSox7 transgenes is shown below the graph. (B) Picture of a blastic colony from replating with doxycycline (×20 magnification). (C) Flow cytometric analysis of pooled colonies grown for 6 days in secondary replating with doxycycline. (D) 5 individual iSox7+ colonies from primary replating of 2 different embryos were replated in secondary clonogenic assay with doxycycline. (E) After May-Grünwald Giemsa staining, erythrocyte and myeloid cells were scored from individual iSox7+ colonies grown for 5 days in liquid hematopoietic conditions without doxycycline. A total of 100 cells were counted per colony. (F) Cells from individual embryos were tested in clonogenic replating assay for hematopoietic progenitors with or without doxycycline. For each embryo, 1/10 of the cells was used for genotyping and the remaining equally divided in −dox and +dox replating conditions. HF indicates headfold. The average numbers for each colony type are presented below the graphs; (n) represents the number of embryo analyzed at each stage of development. For blastic colonies, only embryos positive for rtTA and isox7 transgenes are depicted. All data are representative of at least 3 independent experiments.
Finally, to address the onset of appearance of Sox7-responsive precursors, we analyzed their presence at various stages of gastrulation: PS, NP, and headfold. Precursors forming blastic colonies upon Sox7-enforced expression were observed at all stages analyzed, only in rtTA+ iSox7+ double transgenic mice (Figure 7F). Blastic colonies were already detected at the PS stage before the emergence of definitive precursors and rapidly increased in frequency. Sox7-responsive precursors were detected as early as, or even before primitive erythrocyte precursors, with 3 of the 4 double transgenic embryos at the PS stage giving rise to blastic colonies but no primitive erythrocytes colonies (data not shown). Altogether, these data indicate that hematopoietic precursors responsive to Sox7-enforced expression are present in gastrulating embryos at the onset of hematopoietic specification.
Discussion
The data described in the present study identify Sox7 as a novel and important player in the molecular regulation of hematopoietic specification from mesodermal precursors. We show that Sox7 is transiently expressed during the transition from Flk1+ hemangioblast precursors to CD41+ fully committed hematopoietic progenitors. Knockdown of Sox7 expression during mesodermal commitment severely affect both primitive and definitive hematopoietic precursors as well as endothelial precursors. Furthermore, Sox7-enforced expression maintains the proliferative potential of the earliest committed blood progenitors while preventing their differentiation. The presence of Sox7-responsive precursors was observed both in vitro in differentiating EBs and in vivo in gastrulating embryos. The onset of hematopoietic development in embryos is marked by the formation of hemangioblasts that give rise later on to primitive and definitive restricted blood progenitors.1,45 However, very few hemangioblast (on average 1 to 5) are detected per embryo,3 and their direct differentiation into hematopoietic precursors without previous amplification would not be able to fulfill the need in blood progenitors of the growing embryo. At E7.5, around 40 primitive erythrocyte progenitors are found per embryo, which reaches 350 to 400 by E8.25,9 suggesting the requirement for a proliferative step between hemangioblast and committed precursors. On the basis of the timing of Sox7 expression and its effect upon sustained expression, this proliferative stage may well be controlled by Sox7.
Endothelium and blood are 2 closely related lineages, emerging at the same time and location during early embryogenesis, an observation that indeed led almost a century ago to the hypothesis of a common precursor for these 2 lineages.46 Many genes such as Flk1, Fli1, Tie2, or VE–cadherin are expressed by both lineages early in development or later in established lineages and have been shown to be critical for the specification or maintenance of hematopoietic and endothelium lineages.47,48 Several groups33-35 have recently demonstrated a role for Sox7 in vasculogenesis in a redundant mode with Sox18 by using morpholino knockdown of both genes in zebrafish. In those studies, Sox7 and Sox18 double knockdown critically affected arterial-venous identity but did not seem to impact either the onset of endothelium specification or the expression of the hematopoietic genes Gata1, Scl, and Gata2. However, no detailed analysis was performed to investigate either the onset of blood and endothelium development or the status of early hematopoietic progenitors.
In the present study, we observed a strong reduction in the endothelium potential upon knockdown of Sox7 expression in mesodermal precursors. This decrease in potential appears linked to a decrease in survival affecting both endothelial and hematopoietic potential. It is not clear, however, whether this decrease in potential is fully cell autonomous or not, because it may result from loss of inductive signal mediated by other lineages such as primitive endoderm expressing Sox7.31 Further insight into this question will require the generation of conditional knockout for Sox7 expression in specific lineages. Interestingly, Flk1+ cells expressing Sox7 are not enriched in hemangioblast potential and rapidly generated CD41+ expressing hematopoietic precursors. Furthermore, the precursors maintained in a self-renewing state by Sox7 can give rise to primitive and definitive erythrocytes as well as all myeloid lineages but are devoid of any endothelial potential. Taken together, our data suggest that at the onset of mesodermal specification Sox7 expression is critically required for both hematopoietic and endothelium. Afterward, Sox7 expression may restricts the potential of mesodermal precursors to a hematopoietic fate only, suggesting a possible implication of Sox7 in hematopoietic specification rather than only in proliferation and/or differentiation. The data presented here do not allow us to draw a conclusion about the potential role of Sox7 in hematopoietic specification, but it will be extremely interesting in future experiments to address this hypothesis.
Sox17 was recently identified as a critical regulator of the proliferation of fetal and neonatal hematopoietic stem cells36 ; however, it seems unlikely that Sox7 and Sox17 may simply play redundant functions during embryonic hematopoiesis. Indeed, the first committed hematopoietic precursors expressing significant level of Sox7 are generated around E7.5 of embryonic development in the yolk sac. These progenitors give rise mostly to primitive erythrocytes, then definitive precursors, but they are fully devoid of any hematopoietic stem cell activity.49 In contrast, Sox17 appears to be critical for the proliferation of hematopoietic stem cells that start to be generated in the aorta-gonado-mesonephros region after E10.5 of embryonic development.50 These 2 sets of hematopoietic precursors, generated at different time in embryonic development, have very different potential and, as such, are not likely to be fully controlled by the same sets of genes. Supporting this notion, we found that in contrast to Sox7, the expression of Sox17 was not up-regulated in the Bry-GFP+Flk1+ population but rather down-regulated relative to Bry-GFP+ Flk1− mesodermal precursors (G.L. and V.K., unpublished observation, June 2008).
Our data demonstrate that the sustained expression of Sox7 is sufficient to completely alter the balance between proliferation and differentiation at the onset of hematopoiesis. However, the removal of Sox7-enforced expression fully restores this equilibrium and leads to the efficient differentiation of hematopoietic progenitors. This represent a very attractive characteristic of Sox7 function and might in the future become a powerful molecular tool to allow the expansion of hematopoietic progenitors to be used for potential cell replacement therapy. From a fundamental perspective, it will be very interesting to explore the molecular program that is either maintained or initiated by Sox7 expression in blood progenitors, which might allow us to further dissect and understand the molecular mechanisms critical for self-renewal and/or embryonic hematopoietic specification.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of the laboratory for critical reading of the manuscript, Haydn Prosser (The Wellcome Trust Sanger Institute) for invaluable help with the recombineering technology, and Michael Kyba (Lillehei Heart Institute, University of Minnesota) for the Tet-on inducible Ainv18 ES cell line.
This work is funded by Cancer Research UK, grant number C147/A6058.
Authorship
Contributions: V.K. and G.L. designed and supervised the research project; A.G., A.G.S., M.L., and S.P. designed and performed research; and A.G., A.G.S., V.K., and G.L. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valerie Kouskoff, Paterson Institute for Cancer Research, The University of Manchester, Wilmslow Rd, M20 4BX Manchester, United Kingdom; e-mail: vkouskoff@picr.man.ac.uk.
References
Author notes
*A.G. and A.G.S. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal