Abstract
Abstract 900
Epoetin use has been associated with a higher rate of death in selected groups of cancer and renal patients. The US FDA issued a physician advisory and a black box warning was added to the product labeling in March 2007. In July of 2007 Centers for Medicare & Medicaid Services restricted ESA reimbursement. The intent was to limit initiation and continuation of ESA therapy that could put certain patients at risk for adverse events or deleterious outcomes. We undertook a retrospective analysis of claims data for ESAs (epoetin and darbepoetin) to determine what impact, if any, these warnings had on prescribing behavior.
Medco pharmacy claims data were reviewed for all available forms of ESAs dispensed through our Specialty Pharmacy. Eligible patients participating in the Specialty Pharmacy benefit program, and those known to have cancer, renal disease, or HIV were identified from June 2006 through May 2009. ESA users were defined as those to whom any ESA was dispensed through a pharmacy benefit during the target period. Proportion of ESA users by month per eligible patient population, and number of ESA prescriptions per user were determined over time. Drug cost for ESAs was calculated using average wholesale price (AWP) for all ESAs dispensed to the eligible population.
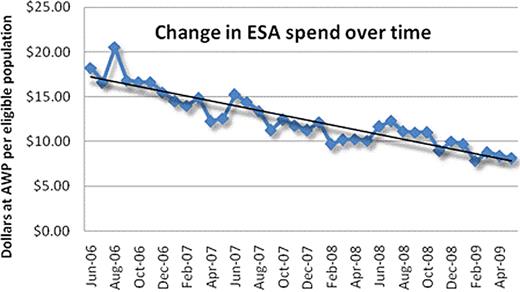
Patients continuously eligible during each 12 month period were 414,905 (6/06 – 5/07), 590,696 (6/07 – 5/ 08), and 731,501 (6/08 – 5/09). Over the 3 year analysis there was an apparent 59% reduction in proportion of eligible patients who received erythropoietin, and a 54% decrease in the number of ESA prescriptions per eligible patient population (coefficient of determination (r2) was 0.87). The number of ESA prescriptions per patient who was prescribed an ESA was not changed over time. Medical claims data, including those for red blood cell transfusions, were available for a subset of the eligible population in this study. In the population for whom these claims were reviewed there was no increase in transfusions, suggesting that the declining use of ESAs was not associated with an increase in the use of transfusions.
The intent of this study was to determine the impact of new guidelines on the prescribing pattern of physicians for ESAs. The findings demonstrate a reduction in the proportion of patients who are prescribed an ESA over the 3 year period, resulting in a significant reduction in the total pharmacy spend for these agents. There was no evidence that the number of prescriptions per patient decreased during the period. This study suggests that physicians have adopted the new health and safety information warnings with ESAs and adjusted prescribing habits.
Lust:Medco Health Solutions, Inc: Employment. Subar:Medco Health Solutions, Inc: Employment. Faris:Accredo Health Group, Inc: Employment. Lin:Medco Health Solutions, Inc: Employment. Weaver:Medco Health Solutions, Inc: Employment. Tully:Medco Health Solutions, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal