Abstract
Abstract 899
The gold standard for determination of the superiority of new treatments is the clinical trial. However, patients in clinical trials tend to be “ideal patients”, i.e. are otherwise healthy except for the condition being examined, have good performance status, may be younger than the average patient with the disease under study, etc, and thus results from clinical trials may not pertain to all patients in the general population. Five year survival for patients with chronic myelocytic leukemic (CML) in recent clinical trials are as high as 95%. However, population based studies of CML show much lower 5-year survival rates. In this study, we compare survival in clinical trials to survival for patients identified from the SEER database as being diagnosed with CML during the same years as the relevant trial was recruiting patients.
We examined survival of patients in randomized controlled trials of treatment of CML between 1980 and 2005 and compared the survival data obtained in these trials to survival of CML patients in the general population of the United States using the Surveillance, Epidemiology, and End Results (SEER) database in the same years as the years of recruitment for the trial. Because age may be a factor in survival, we also calculated age adapted survival for patients in the SEER database for each trial by calculating survival for patients of the same age as the age range given in the trial or for a fifty year interval centered around the median age of patients in the relevant trial.
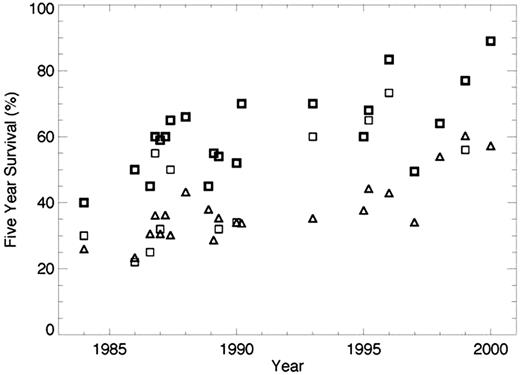
27 trials were identified for data extraction. Median age on clinical trials varied from 37 to 60, whereas the median age of CML patients in the SEER database was 62. The majority of trials recruited patients with chronic phase only. Two trials of patients in accelerated phase were identified. Five year survival on the clinical trials ranged from 30-40% in the earliest trials to 89% for the first trials of imatinib. Five year survival for patients in the general population over the same time period ranged from 22.2% in 1980-87 to 42.7% in 2000-01. Age adapted survival ranged from 26% to 60%. Overall 5-year survival calculated from the SEER database was uniformly lower than survival in clinical trials in the corresponding time period, although age adapted survival overlapped with survival in clinical trials in some cases (see figure). In general, survival from the SEER database was much lower than survival in the corresponding trial for trials of hematopoietic stem cell transplant or interferon and relatively close to that observed in clinical trials after age adaptation for other treatment types.
Survival in clinical trials of treatment for CML is higher than survival of patients with CML in the general population. The difference can be attributed to access to newer medications, a bias toward selecting younger, healthier patients for clinical trials, the requirement in most trials that patients be in the chronic phase of the disease, and time necessary for new treatments identified as superior by clinical trials to be adopted by practitioners. In particular, the difference in survival was larger for trials of more difficult to tolerate treatments such as interferon or stem cell transplantation. This finding underscores the need for population based studies to give a more realistic idea of survival of patients with a given malignancy in the general population. The inclusion of a more diverse patient population in clinical trials, including older and less fit patients, may reduce the disparity.
Five year survival for patients in clinical trials (squares) and age adapted survival for patients in the SEER database (triangles.) In trials in which survival was different between treatment types, the bold squares represent the higher survival value, the lighter the lower survival. Date on the x-axis represents the middle year of recruitment for the trial. When the middle year of recruitment was the same for more than one trial, the values are staggered for clarity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal