Abstract
Abstract 744
Despite enormous progress over the past decade, the “cure” word has been avoided in the context of myeloma therapy. The observation of ∼90% sustained complete response (CR) with Total Therapy 3 (TT3) among the 85% with gene expression profiling (GEP)-defined low-risk disease prompted this investigation of modeling for cure in all Total Therapy (TT) trials.
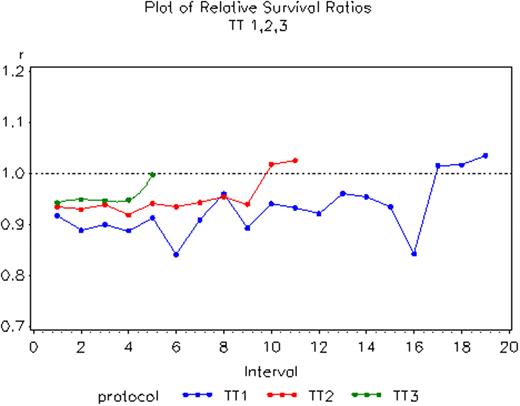
Kaplan-Meier plots of event-free survival (EFS) and complete response duration (CRD) were examined to determine whether cure fractions (CF) could be predicted from the presence of a plateau; in the case of CRD, estimating CF required the multiplication by the CR rate. Median follow-up times with TT1 (n=231), TT2 (n=668) and TT3 (n=303) were 15.7yr, 7.4yr and 4.2yr. CF estimates were examined overall and according to prognostically relevant baseline variables such as cytogenetic abnormalities (CA), available for all trials, and GEP, available to subsets of patients on TT2 and TT3. Relative survival ratios were computed by comparing patient survival with expected survival of a comparable group from the general population.
With TT1, significant CF estimates existed for EFS (6.4%) and CRD (6.0%). With TT2, significant CF estimates were noted for EFS with both control (9.9%) and thalidomide arms (23%). With TT3, CRD-derived CF for low risk patients was high at 55% (p<0.0001). Relative survival ratios revealed progressively earlier normalization of such ratios, apparent at 17yr in TT1, 10yr in TT2 and a remarkably short 5yr in TT3.
Cure is a realistic goal of myeloma therapy whether or not CR was documented as evidenced through long-term follow-up in TT1. The observation of a high CF value of 55% in TT3 for low-risk myeloma with only 4.2yr of follow-up attests to the dramatically greater efficacy of this trial comprising both bortezomib and thalidomide. Therefore, cure should be an objective of contemporary trials for low-risk myeloma while intensive searches for more effective therapy are urged to improve outcomes for high-risk disease.
van Rhee:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal