Abstract
Abstract 684
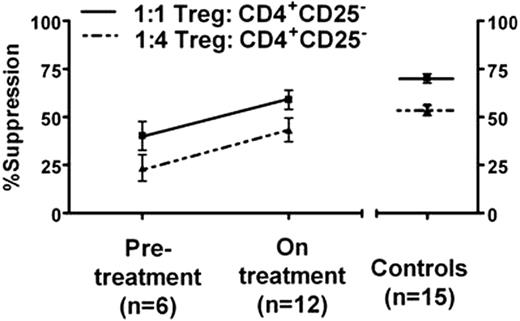
Improved Treg function correlated with reduction in IL-2-producing Th cells (Pearsons r=0.6, p=0.01), and patients on treatment had a 30% reduced frequency single positive type 1 IFN-g-producing Th cells (p=0.04). Circulatory levels of pro-inflammatory sCD40L in platelet poor plasma (PPP) were decreased in patients on treatment versus pre-treatment (0.54 ± 0.05 ng/ml versus 0.89 ± 0.13ng/ml, p=0.02) and comparable to controls (p=0.9). TGF-b levels in PPP strongly correlated with improved platelet counts (Pearsons r=0.8, p<0.0001) and were increased in patients following treatment (2177 ± 258 ng/ml versus 1083 ± 127 ng/ml p=0.004). In summary, our data indicates that treatment with thrombopoietic agents results in improved immune regulation by Tregs which may be responsible for the observed dampening of the pro-inflammatory Th1-associated responses. The concomitant increase in TGF-b levels and decrease in sCD40L levels, both of which are platelet-derived, are consistent with a rise in platelet counts in patients on treatment. This raises the interesting possibility that platelets in patients on treatment may play a role in improving Treg function either directly through cell-cell interactions or indirectly by release of TGF-b. In conclusion, our findings suggest that thrombopoietic agents are not immunologically inert but rather have profound effects to restore immune tolerance.
Bussel:Genzyme: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai, Inc: Research Funding; Sysmex: Research Funding; Scienta: Speakers Bureau; Shionogi: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal