Abstract
Abstract 519
High-dose (hd) therapy with stem cell autograft is an effective treatment for both non-Hodgkin's (NHL) and Hodgkin's Lymphoma (HL). However, the occurrence of secondary malignancies, particularly myelodysplastic syndromes/acute leukemias (sMDS/AL), is a critical issue, representing a major cause of failure in patients potentially cured after hd-chemotherapy.
To evaluate: frequency-cumulative incidence-risk factors, of both sMDS/AL and solid tumors in a large series of lymphoma patients, treated with the hd-sequential (HDS) chemotherapy approach, followed by peripheral blood progenitor cell (PBPC) autograft.
Data have been collected on 1,347 lymphoma patients treated in the last two decades at 11 Centers, associated to GITIL. Patients received either the original or modified HDS regimens; PBPC were collected after hd-cyclophosphamide, and, in a subgroup, after a 2nd round of mobilization with hd-Ara-C. The series included 1,024 B-cell NHL, 234 HL and 89 T-cell NHL; there were 695 high-grade, 278 low-grade and 140 mantle-cell lymphoma; median age was 46 yrs; 57% were male. Overall, 640 (47%) patients received HDS front-line. Autograft was usually performed with PBPC (median CD34+ cells: 7×106/kg); most patients (n=774) received PBPC collected at the 1st mobilization course, whereas some others (n=298) were autografted with PBPC collected following a 2nd high-dose course. The Mitoxantrone/L-PAM combination was the most frequently employed conditioning for autograft (n=643), followed by BEAM (n=301); TBI-conditioning regimen was employed only in 79 patients.
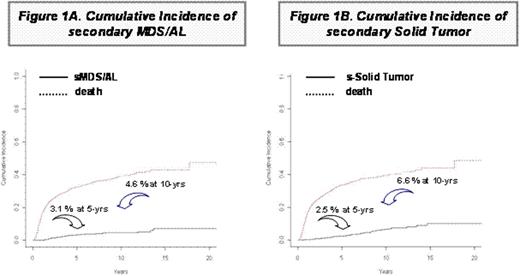
At a median follow-up of 7 yrs, the 5 and 10 yr Overall Survival (OS) projections are 64% and 56%, respectively. Among B-cell NHL, 70.5% of patients treated with Rituximab-supplemented HDS are presently alive vs. 55.6 of those treated with Rituximab-free HDS (p=0.001). Overall, 54 (4.0%) patients developed s-MDS/AL, with a cumulative incidence of sMDS/AL of 3.1% at 5 yrs and 4.6% at 10 yrs. (Figure 1A). Median time of s-MDS/AL occurrence was 3.2 yrs (range: 1.3-13.7) since autograft. In addition, 64 patients had a diagnosis of solid tumor, at a median time of 5.5 yrs (range: 0.5-14.6) since autograft. The cumulative incidence of 2ry-solid tumor is 2.5 % at 5 yrs and 6.6 % at 10 yrs. (Figure 1B). In univariate analysis, age >45 yrs., male sex and autograft with PBPC collected at the 2nd mobilization round had a significantly higher risk of sMDS/AL; a trend for a higher incidence was observed in patients receiving HDS with Rituximab (p=0.066) and in those presenting with BM involvement (p=0.097); on multivariate analysis, male gender and autograft with PBPC of the 2nd mobilization round were the only parameters associated with sMDS/AL occurrence (SDHR: 2.74, p=0.004 and 2.44, p=0.002, respectively). Concerning solid tumors, in univariate analysis, age >45 yrs, Rituximab addition to HDS, HDS with hd-Ara-C, and autograft with PBPC collected at the 2nd mobilization round had a significantly higher risk of solid tumor; on multivariate analysis, age >45 yrs., Rituximab addition and consolidation Radiotherapy following HDS were the only parameters associated with solid tumor occurrence (SDHR: 2.10, p=0.002, 1.99, p=0.011 and 2.13, p=0.024, respectively).
The incidence of sMDS/AL observed following HDS is among the lowest reported so far in lymphoma patients treated with hd-therapy and autograft. However, the risk of solid tumor occurrence can not be ignored. Among risk factors, male gender and the quality, rather than the quantity, of grafted PBPC are the strongest factors associated with sMDS/AL. Other factors, namely older age, Rituximab addition and consolidation Radiotherapy, are mainly associated with the development of solid tumors. Despite the increased risk of secondary solid tumor, addition of Rituximab resulted in significant improvements in the overall survival of patients undergoing HDS. Further analysis are required to understand the biological meaning of the variable association between risk factor and secondary malignancy development.
Tarella:Roche: Honoraria, research financial support. Rambaldi:Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal