Abstract
Abstract 4930
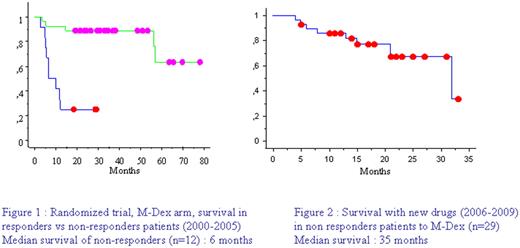
AL amyloidosis is a monoclonal disorder responsible for secretion of a monoclonal free light chain which will deposit as aggregated fibrils and cause organ dysfunction. Prognosis without treatment is poor with median survival around 1 year. Since our multicentric randomized trial comparing M-dex (oral Melphalan and Dexamethasone combination) and high dose treatment with stem cell support (Jaccard et al, NEJM 2007), M-Dex is our reference front line therapy in AL patients, whatever the risk group. Median survival with this strategy has dramatically improved, reaching 5 years in our study as well as in the Italian one (Palladini et al, Blood 2007). Nevertheless survival of refractory patients was poor in the absence of valuable rescue treatment between 2000 and 2005(Figure 1). New drugs as Thalidomide, Lenalidomide or Bortezomib, whose efficacy has been proven in multiple myeloma, has been reported to be effective and tolerable in AL patients. We performed a retrospective multicentric study to determine outcome of M-Dex refractory patients in the era of these new drugs.
Patients with biopsy proven AL amyloidosis, treated with M-Dex, front line, since June 2006 were included if they were considered as refractory by there referent physician in 10 centres belonging to the French network for AL amyloidosis. We recorded the hematological response with second line treatment. Survival was analyzed from the first treatment date using Kaplan Meier model.
We included 29 patients with a median age of 60 years (32-76), 16 patients had cardiac involvement, 19 renal involvement. The median number of organ involvement was 2 (1-5). Isotype of monoclonal light chain was kappa in 38% of cases, and lambda in 62%. Median abnormal free light chain level was 158 mg/L (25.9-2100). Twenty patients (69 %) were considered as non responders because they did not reached a 50% decrease in free light chain serum level and 9 patients (31%), who achieved a partial hematological response, because they did not have a clinical response. The median time between the first M-Dex cycle and the second line treatment was five months (1-17). Second line consisted in thalidomide in 5 patients, lenalidomide in 7 patients, and Bortezomib in 17 patients, in combination with sequential Dexamethasone. Hematological response occurred in 69% of the whole series, with 27% complete response. Depending on treatment, partial hematological response was obtained in 4/5 patients with thalidomide, 2/7 patients with lenalidomide, and 14/17 patients with bortezomib responded with 8 complete responses. With a median follow-up of 21 months (0-32) 69% of patients are alive (Figure 2).
As expected introduction of new drugs for treatment of refractory AL patients gives a high level of hematological response leading to a better survival. Bortezomib seems to be particularly attractive with hematological response rate of 82%, and 47% complete response. The combination of M-Dex and bortezomib will be compared soon with M-Dex in a prospective international multicentric study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal