Abstract
Abstract 4293
The major cause of failure after allogeneic hematopoietic stem cell transplantation (SCT) for acute lymphoblastic leukemia (ALL) is disease relapse.
We analyzed the outcome of second SCT for treatment of disease relapse occurring within 6 months (n=10) or after 6 months (n=18) following first SCT in 28 ALL patients (12F/16M) with median age 23.5 years (range 3-50) transplanted between 1993 and 2009 at our institution. At time of SCT 43% of patients had advanced remission beyond CR1 (n=12) and 57% had active disease (n=16). High risk cytogenetic profile defined by the presence of t(9;22) or t(4;11) translocation, or hypodiploid clone was noted in 43% of patients (n=12) at time of diagnosis. A myeloablative busulfan- or TBI-based transplant preparative regimen (n=8) or reduced-intensity fludarabine-based regimen (n=20) was used followed by matched sibling (n=17), matched unrelated (n=9), or mismatched cord blood (n=2) infusion. A different donor was used in 54% of cases. GVHD prophylaxis was either tacrolimus-based (n=20) or cyclosporine-based (n=8). Only one patient in this analysis received consolidation following second SCT in the form of prophylactic donor lymphocyte infusion (DLI).
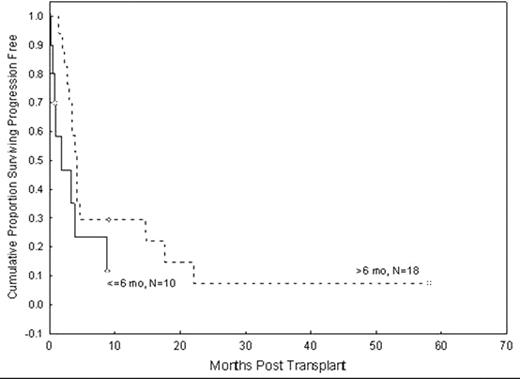
26 patients were evaluable for response with 2 early deaths within 30 days of SCT. Overall response rate was 88% with 38% sustained (n=10) and 50% complete (n=13) response rates. Acute grades II-IV and III-IV GVHD rates were 27% (n=7) and 12% (n=3), respectively; chronic extensive GVHD was noted in 1 patient. Progression-free (PFS) and overall survival (OS) were 27% and 42%, respectively, at 1 year. However, there is only one long-term survivor at 5 years, with a median follow-up time of 6 months among 5 survivors. The primary cause of death was disease relapse (n=14), with remaining causes GVHD (n=4), infection (n=2), multi-organ failure (n=2). Disease characteristics, including WBC count and cytogenetic profile at diagnosis, time to first remission, time to progression after first SCT, time between first and second SCT, age at SCT, intensity of SCT preparative regimen, disease stage at SCT, donor relation, use of same or different donor, and development of GVHD were evaluated in a univariate analysis for PFS following second SCT without any statistically significant factors identified. There was a trend for longer PFS in patients who had a late relapse following first SCT (p=.06). At 1 year, PFS was 29% for patients whose disease progressed after 6 months following first SCT versus only 12% for those who had very early relapse (p=.06, Figure 1).
In summary, a second allogeneic SCT remains an effective method for achieving response in a highly refractory patient population. However, post-SCT interventions are needed to achieve sustained response, as demonstrated by the long-term remission of the only patient who received post SCT prophylactic DLI. A significant proportion of patients remain disease-free up to one year following second SCT, thereby providing a window of time to administer consolidation in the form of immunotherapy or low-dose chemotherapy maintenance.
Progression-free survival following second transplant in patients with a short (≤6 months) versus longer (> 6 months) PFS following the first transplant.
Progression-free survival following second transplant in patients with a short (≤6 months) versus longer (> 6 months) PFS following the first transplant.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal