Abstract
Abstract 4062
Poster Board III-997
Over 70% of deaths in regularly transfused patients with β-thalassemia major (TM) are related to iron-induced cardiomyopathy. Deferasirox (Exjade®), in a sub-study of the 1-year multicenter prospective EPIC trial, demonstrated efficacy in reducing myocardial iron in TM patients with mild, moderate and severe cardiac siderosis, as evidenced by a statistically significant improvement in myocardial T2*. We herein report the extension phase results from the same study in patients who have received up to 2 years of deferasirox therapy.
Patients aged ≥10 years with myocardial T2* >5–<20 ms (indicating cardiac siderosis) by cardiovascular magnetic resonance (CMR), left ventricular ejection fraction (LVEF) ≥56%, serum ferritin >2500 ng/mL, MR (R2) LIC >10 mg Fe/g dry weight (dw), and a lifetime minimum of 50 transfused blood units were included in the cardiac sub-study. Deferasirox was initiated at 30 mg/kg/day and increased to 40 mg/kg/day by the time patients had entered the 1-year extension. Dose decreases were allowed for safety reasons. The primary endpoint was change in myocardial T2* from baseline to 2 years.
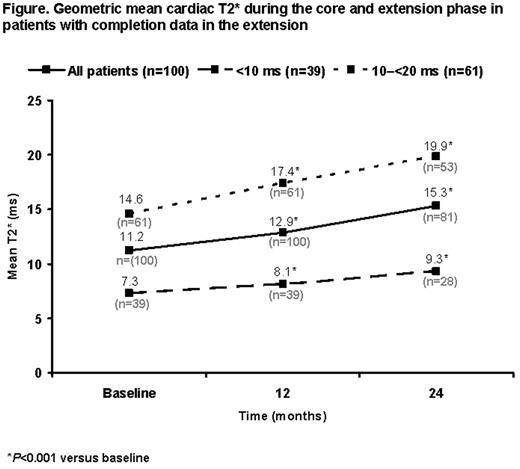
Out of 100 patients who entered the 1-year extension phase, 85 completed (85%); 24-month CMR data are available for 81 patients. Mean age was 20.6 ±7.3 years. Baseline cardiac T2* was <10 ms (severe cardiac siderosis) in 39 patients (39%) and 10–20 ms (mild-to-moderate cardiac siderosis) in 61 (61%). 67.0% had received prior deferoxamine (DFO) and 33.0% prior DFO/deferiprone combination therapy. Mean actual deferasirox dose increased from 33.1 ±3.7 mg/kg/day in the core 1-year phase to 36.1 ±7.4 mg/kg/day during the extension. Continued improvement in myocardial T2* was observed in the extension phase so that after 2 years of deferasirox treatment, T2* had significantly increased from a baseline geometric mean of 11.2 to 15.3 ms (P<0.001). Significant increases from 7.3 to 9.3 ms (P<0.001) and from 14.6 to 19.9 ms (P<0.001) were respectively noted in patients with baseline T2* <10 and 10–20 ms (Figure). LVEF remained stable in both subgroups throughout the 2-year follow up period. Both mean LIC and median serum ferritin were significantly reduced from baseline by 10.7 ± 12.8 mg Fe/g dw and 2343 ng/mL (range –12795 to 25127), respectively (P<0.001; based on last-observation-carried-forward analysis).
Reasons for discontinuation were: unsatisfactory therapeutic effect (n=8), consent withdrawal (n=3), protocol violation (n=2), lost to follow up (n=1) and abnormal laboratory value (increased urinary protein/creatinine ratio) leading to consent withdrawal (n=1); no deaths were reported. Incidence of investigator-assessed drug-related AEs (≥5%) decreased overall from the core phase to the extension: increased blood creatinine (n=21 [21.0%] vs n=18 [18.0%]), rash (n=15 [15.0%] vs n=0), increased alanine aminotransferase (ALT) (n=6 [6.0%] vs n=4 [4.0%]) and increased aspartate aminotransferase (n=4 [4.0%] vs n=3 [3.0%]). There were no drug-related serious AEs over 2 years. In total, 4 patients (4.0%) had increased serum creatinine >33% above baseline and the upper limit of normal (ULN) on two consecutive visits; 3 patients (3.0%) during the core and 1 (1.0%) during the extension. 4 (4.0%) patients had increased ALT >10xULN on two consecutive visits; 2 patients (2.0%) during the core and 2 (2.0%) during the extension; levels were already >ULN at baseline in these patients.
This is the first large prospective study to report 2-year data on cardiac iron removal for any iron chelator. Results show that continued therapy with deferasirox for up to 2 years at doses 30–40 mg/kg/day was effective in removing iron from the heart in TM patients with mild, moderate and severe cardiac siderosis. Myocardial T2* continued to improve in year 2 and the statistically significant improvement from baseline was associated with maintenance of normal cardiac function and a concomitant decrease in hepatic and total body iron burden. Overall, deferasirox was well tolerated.
Pennell:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Apopharma: Consultancy, Honoraria; Cardiovascular Imaging Solutions: Equity Ownership; Siemens: Research Funding. Off Label Use: THE SPECIFIC USE OF CHELATION FOR CARDIAC SIDEROSIS IS OFF-LABEL. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Vifor International: Membership on an entity's Board of Directors or advisory committees. Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genzyme: Membership on an entity's Board of Directors or advisory committees. Chan:Novartis: Honoraria, Research Funding. Aydinok:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Ibrahim:Novartis: Research Funding. Li:Novartis: Consultancy, Speakers Bureau. Viprakasit:Thai Government : Employment; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Government Pharmaceutical Organization of Thailand: Honoraria, Research Funding. Kattamis:Novartis: Consultancy, Honoraria, Speakers Bureau. Smith:Novartis Pharma AG: Consultancy, Employment at Royal Brompton Hospital funded by Novartis Pharma AG. Habr:Novartis Pharmaceuticals: Employment. Domokos:Novartis Pharma AG: Employment. Roubert:Novartis Pharma AG: Employment. Taher:Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal