Abstract
Abstract 3804
Poster Board III-740
Epigenetic therapy with a hypomethylating agent is becoming the standard of care in some intermediate and high-risk Myelodysplastic Syndromes (MDS) and Chronic Myelomonocytic Leukemia (CMML).
This multicenter, open label, single-arm study evaluated the efficacy and safety of the 5-day decitabine (DACOGEN, Janssen Cilag Farmaceutica S.A. and Eisai Inc.) dosing regimen in patients with MDS and CMML on a “Real World Program”.
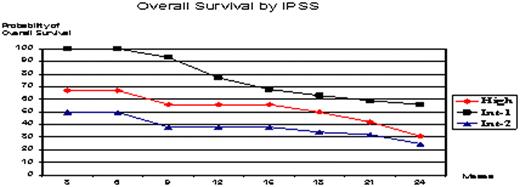
Eligible patients were enrolled at different sites from South Korea and Argentina. A report prepared ad-hoc was completed. WHO classification was taken into account, as well as International Prognostic Scoring System (IPSS), performance status by ECOG, co-morbidities, previous treatments and IWG 2006 criteria. Efficacy was evaluated with at least 2 cycles. Inclusion Criteria: ≥18 years of age; de novo or secondary MDS; all WHO Subtypes and CMML type 1 and type 2. Exclusion Criteria: Acute Myeloid Leukemia (AML) or other progressive malignant disease. All patients received decitabine 20 mg/m2 IV over 1 hour x 5 days, with cycles repeated every 4 weeks.
One hundred sixteen patients enrolled (Intend-To Treat) and 99 were evaluable: median age 64, 73% male, 93% de novo MDS, median time from diagnosis 9 months, and 19% with prior chemotherapy treatment. WHO classification was: RA 1%; RARS 2%; RCMD 25%; RCMDRS 4%, RAEB-1 12%; RAEB-2 28%; MDSu 1%; CMML type-1 17% and type-2 9%. IPSS: High (17%), Int-2 (25%), Int-1 (55%) and Low (3%). Co-morbidities were described in 51%. This report includes data from a 24-month follow-up period. Patients received a median of 5 cycles (range 1-13), with 42% receiving ≥6 cycles. Overall improvement rate in the evaluable population was 35% by IWG 2006 criteria (19% CR + 4% mCR + 4% PR + 8% HI) and the rate of stable disease or better was 50%. Median time to first and best response was 2.2 and 3.9 months respectively. 37% of patients died during study period. Adverse events attributed to the study drug were febrile neutropenia (59%), thrombocytopenia and bleeding (18%), asthenia (30%), fatigue (12%), and eosinophilia (4%). Three patients received an Allo Stem Cell Transplant after achieving CR and did well. Two percent of patients responded after cycle 4. Four patients withdrew decitabine after getting CR, they relapsed and re-treatment was successful. Twenty-one percent of patients developed AML during follow-up.
Decitabine showed a prompt clinical activity and the overall improvement rate was 35%. This drug had a manageable toxicity profile. Previous chemotherapy treatment was an unfavorable risk factor. Although delayed response has been observed (2%), no less than 4 cycles should be given. Relapse was a rule after withdrawing treatment, so keeping dosing and interval is really important. This treatment was very active in CMML and allowed patients to be transplanted in a better condition.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal