Abstract
Abstract 3803
Poster Board III-739
Transfusional iron overload can cause hepatocellular injury, which manifests as increased liver transaminase levels and, in some cases, may progress to cirrhosis. The once-daily oral iron chelator deferasirox (Exjade®) has been shown to reduce iron overload in patients with various transfusion-dependent anemias, and a previous analysis demonstrated a correlation between decreased iron overload and improved transaminase levels [Brissot P et al. Blood 2006;108(11):abst 3817]. As liver dysfunction is a common complication in patients with myelodysplastic syndromes (MDS), this analysis evaluates the relationship between serum ferritin (as a marker of iron overload) and alanine aminotransferase (ALT; as a marker of liver function) during deferasirox treatment in a large MDS population.
This analysis is based on data from iron overloaded MDS patients aged ≥2 years who were enrolled in the 1-year multicenter EPIC study; patients with a life expectancy <1 year were excluded. Patients initially received deferasirox 10–30 mg/kg/day, depending on the frequency of blood transfusions; 5–10 mg/kg/day dose adjustments (range 0–40 mg/kg/day) were made based on 3-month serum ferritin trends and safety markers. Serum ferritin and ALT were assessed monthly by standard blood chemistry examinations.
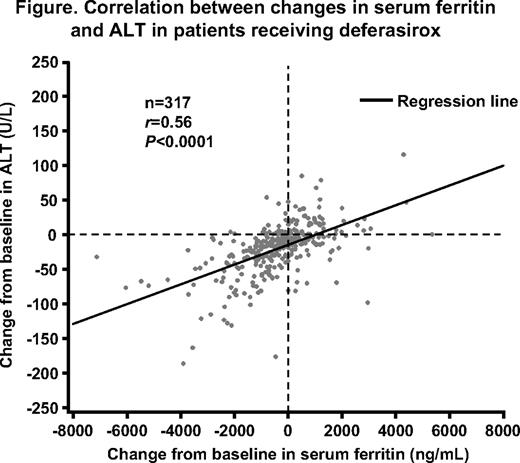
In total, 341 patients with MDS (mean age 67.9 ± 11.4 years) were enrolled in the EPIC study. Mean actual deferasirox dose during treatment was 19.2 ± 5.4 mg/kg/day. Median serum ferritin at baseline, 3, 6, 9 and 12 months was 2730, 2358, 2210, 2076 and 1904 ng/mL, respectively, and the median change from baseline was significant at 12 months (P=0.0019). Mean ALT at the same time points was 59.7, 48.4, 42.0, 40.5 and 36.1 U/L, respectively; mean change from baseline in ALT at 12 months was also significant (P<0.0001; n=169). A significant correlation was observed between absolute change in serum ferritin and ALT from baseline to month 12 (P<0.0001; Figure), indicating that a mean decrease in serum ferritin of 500 ng/mL was associated with a mean decrease in ALT of 21.6 U/L.
In these chronically transfused MDS patients, deferasirox treatment produced a significant decrease in serum ferritin during 1 year of treatment. Improvements in ALT, which is an important indicator of hepatocellular injury, mirrored the reductions in serum ferritin and these changes were significantly correlated. Additional prospective studies are warranted to further investigate this association between iron burden and liver dysfunction in MDS.
Gattermann:Novartis: Honoraria, Participation in Advisory Boards on deferasirox clinical trials. Habr:Novartis Pharmaceuticals: Employment. Domokos:Novartis Pharma AG: Employment. Roubert:Novartis Pharma AG: Employment. Fenaux:Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Ortho Biotech: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Cephalon: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal