Abstract
Abstract 3730
Poster Board III-666
Histone deacetylase (HDAC) inhibition has emerged as a promising therapeutic approach in malignancies. HDAC inhibition has proved to be a particularly effective option in patients with lymphoma. The HDAC inhibitor vorinostat is approved for the treatment of patients with cutaneous T-cell lymphomas and is being tested in patients with B cell lymphomas. More recently, a number of other HDAC inhibitors have entered preclinical and clinical testing.
The mechanisms through which HDAC inhibitors exert their downstream effects are currently unknown. As the number of HDAC inhibitors in development increases, it is unclear if they share a class effect or display unique mechanisms of action. Recently, LBH589 has been described as an orally available, highly potent inhibitor of HDAC. We decided to explore whether LBH589 would be an effective therapeutic option for patients with lymphoma.
In order to evaluate whether LBH was efficacious and potent in B cell lymphomas, we tested both vorinostat and LBH589 in the same cell line(s). We found that LBH589 was over 10 times more potent than vorinostat (mean IC50 7.4nM versus 830nM). We decided to further test LBH589 in an expanded panel of 18 cell lines derived from 5 different lymphoid malignancies: Burkitt lymphoma, mantle cell lymphoma, Hodgkin lymphoma, multiple myeloma and diffuse large B cell lymphoma. LBH589 was found to be lethal in each of these cell lines at IC50 concentrations varying from 5.6-31.5 nM (mean 11.2nM), suggesting that this drug may be effective at physiologically achievable concentrations.
Based on the IC50 cut-off of 10nM, we assigned the treated cell lines to 2 groups: highly sensitive (IC50 < 10nM, N=11) and less sensitive (IC50> 10nM, N=8). We performed gene expression profiling on 12 of these cell lines and compared the gene expression profiles of the highly sensitive versus less sensitive cell lines. Further, we performed time course experiments in which we evaluated the effects of LBH589 at its IC50 on cell lines at 6 and 12 hours post-treatment. Gene expression profiling was performed on the treated cells at each time point. We also engineered resistant cell lines by incremental dose escalation over a period of months to a concentration greater than or equal to the IC50. The resistant cell lines were also profiled for gene expression and compared to the wild type cell lines.
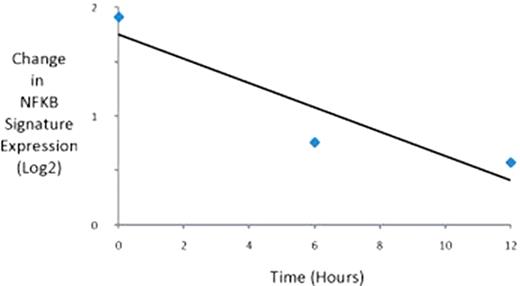
The gene expression profiles of LBH589 treated cells at 6 and 12 hours demonstrated a clear and progressive down regulation of genes associated with the NF-KB pathway (Figure 1). Furthermore, cell lines with high expression of genes in the NF-KB pathway were uniformly highly sensitive to LBH589 with IC50<10nM in all cases.
NF-KB activation is a common feature of many different lymphoma types. Our data suggest that HDAC inhibition using LBH589 could provide a potent method for treating lymphomas and that HDAC inhibitors may exert their effects through the down-regulation of the NF-KB pathway. Our data also suggest a rationale for dual inhibition of HDAC and NF-KB in the treatment of lymphoma.
Rizzieri:Merck & Co., Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal