Abstract
Reducing transplant-related mortality and facilitating donor engraftment are very important goals in hematopoietic cell transplantation (HCT). Plerixafor (Genzyme, Cambridge, MA), previously called AMD3100, is a highly specific and reversible antagonist of CXCR4. It was recently approved by the FDA in combination with granulocyte-colony stimulating factor (G-CSF) for stem cell mobilization. Herein, we reasoned that plerixafor administered after transplantation could increase donor stem cell homing efficiency by increasing the availabel marrow niche microenvironment. We hypothesized that the availabel niches would be increased by plerixafor-induced mobilization of the recipient's residual bone marrow cells from marrow niches, allowing a competitive advantage for the healthy donor cells.
KTLS cells (i.e., c-Kit+ Thy1low Lin- Sca-1+ hematopoietic stem cells) obtained from C57BL/Ka CD45.1 Thy1.1 mice, were injected intravenously into lethally (10.5 Gy) irradiated C57BL/6 CD45.2 Thy1.2 mice (250 KTLS cells per recipient mouse). Beginning at day +2 post transplant, the mice were injected subcutaneously with PBS mock control or plerixafor at 5mg/kg every Monday, Wednesday and Friday up to day +56. Animal survival and hematological recovery were determined. The stimulating effects of plerixafor on marrow cells were measured in vitro using thymidine incorporation assays and colony-forming unit (CFU) assays. Percent spleen seeding efficiency of donor stem cells was calculated by dividing the absolute number of CD45.1+ KTLS stem cells in the spleen by the number of injected KTLS stem cells. To determine stem cell mobilization by plerixafor, the recipient-origin stem cells (CD45.2+ c-kit+ Lin- Sca-1+ cells) in the marrow were quantified.
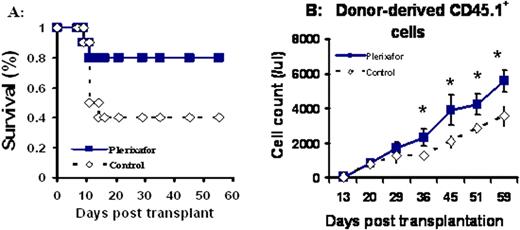
We found that post-transplant administration of plerixafor significantly improves animal survival (Fig. A). Approximately 60% of transplanted mice receiving PBS control died within 2 weeks following transplantation, whereas only 20% of transplanted mice treated with plerixafor died. Additionally, plerixafor selectively enhanced donor cell engraftment and promoted the recovery of all lineages of cells including T and B cells, red cells and platelets. A representative donor derived CD45.1+ blood cell recovery was shown in Fig. B (*: P < 0.01). Furthermore, compared to PBS injected control mice, mice receiving plerixafor had increased frequencies of marrow CFUs-GM and a much denser and more cellular marrow at day +65. Unlike G-CSF, plerixafor has minimal proliferating effects. Finally, we found that plerixafor mobilized recipient stem cells and increased the homing of donor stem cells to hematopoietic sites. The seeding efficiency of donor KTLS stem cells to the spleen was 6.4±1.5% in plerixafor treated mice, compared to 1±0.4% in PBS control mice (p <0.01). The marrow of plerixafor-treated mice had significantly higher number of hematopoietic stem/progenitor cells as measured by CFUs-GM and BFUs-E compared to PBS injected control mice (p<0.05).
Plerixafor mobilizes recipient residual stem cells from niche microenvironments and increases the access of niches for donor stem cells. This results in a selective enhancement of donor cell engraftment. Our study indicates that plerixafor represents a new approach for enhancing hematological recovery following HCT.
Kang:Genzyme: Research Funding. Chao:Genzyme: Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal