Abstract
Abstract 35
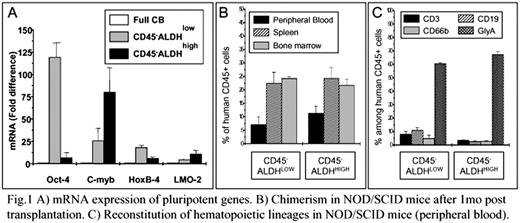
Recently, we identified in umbilical cord blood (UCB) a population of very small embryonic/epiblast-like (VSEL) stem cells (Leukemia 2007;21:297–303) that are i) smaller than erythrocytes, ii) SSEA-4+/Oct-4+/CD133+/CXCR4+/Lin−/CD45−, iii) respond to SDF-1 gradient and iv) possess large nuclei containing primitive euchromatin. We have demonstrated in vitro that UCB-derived VSELs did not reveal hematopoietic activity freshly after isolation, but grow hematopoietic colonies following co-culture/activation over OP-9 cells. To investigate the hierarchy of UCB-derived, CD45 negative VSELs, we employed staining with Aldefluor - detecting aldehyde dehydrogenase (ALDH), the enzyme expressed in primitive hematopoietic cells. Subsequently, we sorted CD45−/CD133+/ALDHhigh and CD45−/CD133+/ALDHlow sub-fractions of VSELs from UCB samples and established that freshly sorted from UCB VSELs in contrast to sorted CD45+/ CD133+/ALDHhigh and CD45+/CD133+/ALDHlow hematopoietic stem cells (HSC) did not grow colonies in vitro. However, when CD45− VSELs were activated/expanded over OP-9 stroma cells, they exhibit hematopoietic potential and grew in routine methylcellulose cultures hematopoietic colonies composed of CD45+ cells. Interestingly, while CD45−/CD133+/ALDHhigh VSELs gave raise to hematopoietic colonies after the first replating, the formation of colonies by CD45−/CD133+/ALDHlow VSELs was somehow delayed, what suggest that they needed more time to acquire hematopoietic commitment. Thus our in vitro data indicate that both populations of CD45− cells may acquire hematopoietic potential; however hematopoietic specification is delayed for CD45−/CD133+/ALDHlow cells, suggesting their more primitive nature. In parallel, real time PCR analysis confirmed that while freshly isolated CD45−/CD133+/ALDHhigh VSELs express more hematopoietic transcripts (e.g., c-myb, 80.2±27.4 fold difference), CD45−/CD133+/ALDHlow exhibit higher levels of pluripotent stem cell markers (e.g., Oct-4, 119.5±15.5 fold difference as compared to total UCB mononuclear cells) (Figure 1 panel A). Next hematopoietic potential of UCB-derived VSELs was tested in vivo after transplantation into NOD/SCID mice (Figure 1 panel B and C). We noticed that both CD45−/CD133+/ALDHhigh and CD45−/CD133+/ALDHlow VSELs, give rise to human lympho-hematopoietic chimerism in lethally irradiated NOD/SCID mice as assayed 4–6 weeks after transplantation. The level of human hematopoietic CD45+ cells in murine peripheral blood (PB), bone marrow (BM) and spleen (SP) were comparable for both transplanted UCB-VSELs fractions - 7.1±2.9% (PB), 23.2±0.2% (SP) and 25.2±1.0% (BM). In conclusion, our data suggest that freshly isolated very small CD45 negative UCB-VSELs are depleted from clonogeneic progenitors, however they are highly enriched for primitive HSC. Based on our in vitro and in vivo data we postulate following hierarchy of hematopoietic stem cells in UCB (from most primitive to more differentiated) i) CD45−/CD133+/ALDHlow, ii) CD45−/CD133+/ALDHhigh , iii) CD45+/CD133+/ALDHlow and iv) CD45−/CD133+/ALDHhigh. We also postulate that as we have already shown for murine BM-derived VSELs, human UCB-derived CD45 negative VSELs correspond to a population of most primitive long term repopulating HSC (LT-HSC). Of note, we also found that currently employed, routine UCB processing strategies may lead up to ∼50% unwanted loss of these small cells that are endowed with such remarkable hematopoietic activity!
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal