Abstract
Abstract 3401
Poster Board III-289
Systemic amyloid light chain amyloidosis (AL) is a fatal monoclonal plasma cell disorder which leads to depositions of insoluble fibrils in different organs. Treatment with conventional chemotherapy results in low numbers of complete remission (CR). High-dose melphalan (HDM) with autologous haematopoietic stem cell transplantation (SCT) induces the highest rates of CR (up to 40%) which is a prerequisite for longterm survival. An attempt to further increase CR rate might be the administration of induction therapy (Perz et al., BJH 2004).
From 2003 until 2008, a prospective phase II trial has been performed in our institution. The patients were treated with sequential therapy of 1-3 courses of pulsed dexamethasone (dexa, 40 mg), mobilization chemotherapy with cyclophosphamide 1g/m2, adriamycin 60mg/m2 and dexa followed by HDM and auto-SCT. Exclusion criteria were age > 70 years, symptomatic heart involvement > NYHA stage II and WHO performance status >2. The primary objective of the study was efficacy (CR rate) and tolerability of the treatment. It was planned to include 46 patients in a three step design (Chen 1997). Until October 2008 thirty patients with newly diagnosed AL (median age 55 years; median number of involved organs 2; involved free light chains in serum median 117 mg/l; cardiac Mayo staging system (Dispenzieri et al., JCO 2004) median stage 1) were included. Because patient recruitment was too slow the study was stopped.
The median follow-up for survival according to Korn (1986) of all pts is 41 months after study inclusion. The follow up of the last patient is 4 months after HDM. In the first 5 pts 3 toxic deaths occurred during dexa. After amendment of antibiotic prophylaxis no further treatment-related deaths were observed. Other serious adverse events were arterial thromboses in 2 pts with nephrotic syndrome and sepsis with renal failure in 1 pt. Five pts stopped dexa due to toxicity before completing the planned 3 cycles. Four pts could not be transplanted due to AL progression. Overall, 23 patients have been transplanted.
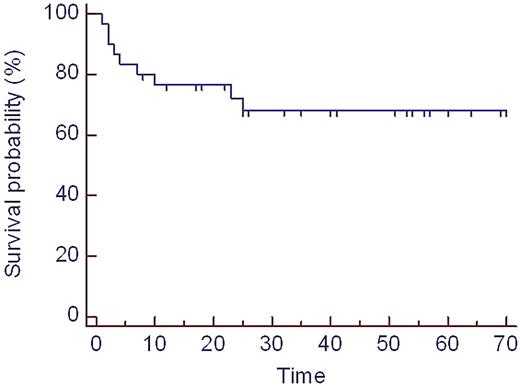
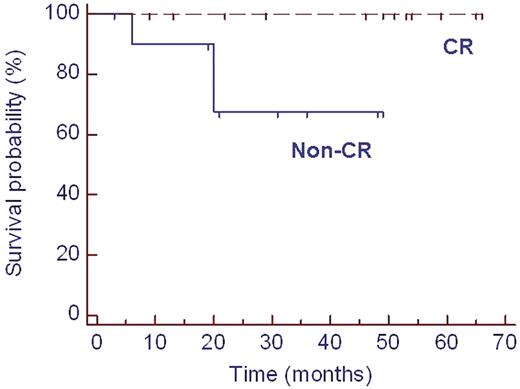
Hematological response was induced in 13 out of 25 (52%) evaluable pts (CR n=4) following induction treatment. After HDM CR was obtained in 12 / 23 (52%) and partial remission in 10 / 23 (43%). This led to organ response (OR) in 13 out of 23 (57%) evaluable patients. No patient died of transplant-related mortality. Overall, 6 pts died due to AL progression. Hematological relapse occurred in 2 pts with CR and progression in 5 pts with PR. Median overall survival (OS) for the whole group is not reached. The last death occurred at 25 months after inclusion with a resulting estimated survival probability of 68% at that time point (figure 1). OS was significantly improved after HDM in those patients who achieved CR compared to patients without CR (p=0.05, figure 2).
Induction therapy with pulsed dexa prior HDM is feasible and effective, but has remarkable toxicity. CR and OR rates after HDM can be increased with this intensive therapy approach compared to historical controls. Furthermore, pts with CR have a low risk of relapse and have an excellent survival. Further improvement regarding toxicity and CR rates might be achieved using lower dosage of steroids and incorporation of the new drugs in the induction therapy prior HDM.
Overall survival (whole study population, n=30) since study inclusion, time in months
Overall survival (whole study population, n=30) since study inclusion, time in months
Overall survival of 23 patients after HDM. CR: complete remission. HDM: high-dose melphalan.
Overall survival of 23 patients after HDM. CR: complete remission. HDM: high-dose melphalan.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal