Abstract
Abstract 3285
Poster Board III-1
Tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL oncoprotein are the current standard care of patients with CML. They also inhibit off-target kinases (e.g. c-KIT, TEC, BTK, PDGFR, SRC), some of which may have important physiological functions in immune responses. Recently, several papers have indicated a significant suppressive effect of TKIs on T- and NK-cell activation and cytotoxicity in vitro. The aim of this study was to assess the effects of TKIs on immune effector cells in patients with CML in chronic phase.
We collected 88 peripheral blood (PB) and 73 bone marrow (BM) samples from 54 CML patients at the time of diagnosis (n=19/17; PB/BM), during imatinib (n=40/39), and dasatinib (n=29/17) therapies. In addition, 16/16 (PB/BM) samples were analyzed from 17 healthy controls. The median time on imatinib or dasatinib therapy was 11 months in both groups (ranges 4-29 and 3-42 months, respectively). Lymphocyte subtypes (T, B, NK and NKT-cells) and regulatory cells (regulatory T-cells and dendritic cells) were analyzed with an extensive flow cytometry panel including key lymphocyte markers for activation (CD57, HLA-DR), proliferation (Ki67), differentiation (CD4, CD8, CD19, CD34, TCR alpha/beta, TCRgamma/delta) and memory status (CD45RA, CD45RO, CD62L).
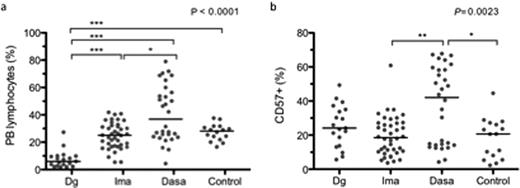
At CML diagnosis, the characteristic findings were a lower B-cell (immature CD34+CD19+ B-cells in particular) and increased NKT proportion of lymphocytes in the BM. Further, a larger proportion of BM CD3+ T-cells expressed CD57. Both in PB and BM the proportions of myeloid and plasmacytoid dendritic cells were decreased at diagnosis. There were no significant differences in proportion of regulatory T-cells (Tregs) from CD4+ cells (all compared to healthy controls, p<0.05). During imatinib therapy, all these changes normalized. The distribution of lymphocyte subclasses and the proliferation and activation status of lymphocytes in imatinib patients did not differ from healthy controls. The only imatinib-specific aberrations observed were a lower number of PB T-cells carrying the gamma-delta T-cell receptor (TCR) chain and a larger amount of CD45RO positive CD4+ T-cells in PB as compared to controls. Dasatinib treated patients differed more markedly from healthy controls and from imatinib. The absolute numbers and proportions of monocytes were increased both in PB and BM, proportions of B-cells were decreased in PB and BM, and proportions and absolute numbers of CD8+ T-cells expressing activation markers HLA-DR and CD57 were increased in PB (p<0.05). When compared to imatinib patients, dasatinib use was associated with a significantly lower Treg proportion in PB (5.7 vs. 2.7% of CD4+) and BM (5.5 vs. 2.8%, p<0.001 for both). Remarkably, dasatinib patients were clearly divided into two distinct equal-sized groups: the other group (“normal”) resembled that of imatinib patients and healthy controls while the other group (“immunoactivated”) was characterized by elevated CD8+ lymphocyte, NK and NKT cell counts in PB (fig.a). Lymphocytes (CD4+, CD8+) of the latter group strongly expressed CD57+ (fig.b), HLA-DR and CD45RO and had lower levels of CD62L antigen conforming to a phenotype of a late memory cytotoxic lymphocyte characteristic of e.g. latent CMV infection. No clinical signs of immunosuppression, such as opportunistic bacterial, viral or fungal infections, were observed in any of the patients.
Character count: 2983
TKIs had a significant and differential off-target effect on the numbers and proportions of immune effector cells. No signs of TKI-therapy related immunosuppression was seen and in particular, a subgroup of patients on dasatinib therapy displayed an immune-activated cellular profile putatively linked to Treg deficiency, unknown individual (genetic) host factors and recurrent antigen exposure. These results underline the importance of careful, long-term surveillance for adverse or off-target effects, which could be restricted only to a subgroup of patients. In addition, our data suggests that TKIs could be used to modulate immune responses in various malignant and non-malignant disorders related to immune dysfunction.
Porkka:BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Mustjoki:BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal