Abstract
Abstract 3022
Poster Board II-998
Dendritic cells (DC) are “professional” antigen-presenting cells (APC) that can prime T cells. Their characteristic morphology and phenotype segregate them from other APC. Many studies suggest that mature DC are able to induce potent antitumor T cell immunity that can reject tumors. Based on this, numerous cancer vaccine trials using ex vivo generated DC have been conducted in humans. However, the observed objective response rates in these studies have been disappointing. This could partially be attributed to difficulties in generating large numbers of clinical grade, optimally matured DC. Also, it is widely accepted that the quality and quantity of DC generated ex vivo vary substantially among individuals. We have hypothesized that the generation of standardized artificial DC (aDC) will overcome the time, expense, and suboptimal reproducibility of DC cultures and prompt the development of DC-based immunotherapy for cancer.
Previously, we developed a renewable and standardized artificial APC (aAPC) by transducing HLA-A2, CD80, and CD83 to the human erythroleukemic suspension cell line, K562. This aAPC can naturally process and present HLA-A2-restricted peptides and uniquely support the priming and prolonged expansion of large numbers of antigen-specific CD8+ CTL. Generated antigen-specific CTL display a central ∼ effector memory phenotype consistent with in vivo persistence, possess potent effector function, and specifically recognize tumor cells. Furthermore, CTL can be maintained in vitro for a prolonged period of time up to >1 year without any feeder cells or cloning. Recent clinical trials have demonstrated that adoptive transfer of anti-tumor CTL with a memory phenotype generated ex vivo using this aAPC, IL-2, and IL-15 can persist in cancer patients as memory T cells for >6 months without any lymphodepletion, adjuvants, or cytokine administration. Clinical responses have also been observed in some patients.
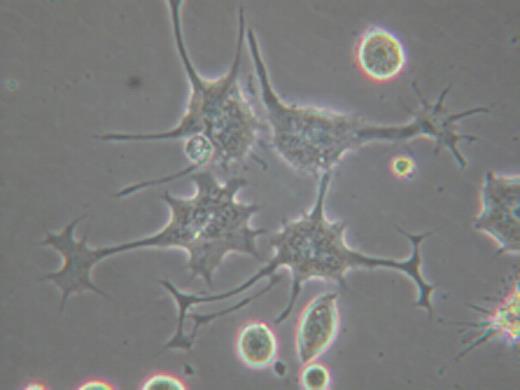
To develop a standardized aDC, we have undertaken an approach to differentiate our K562-based aAPC into aDC. In neurogenesis, it has been well established that a family of Rho GTPases (Rho, Rac, and Cdc42) critically regulates the outgrowth of neurites, i.e. dendrites and axon. We have found that the inhibition of Rho kinase (ROCK), which is a key effector molecule of Rho, can promote the differentiation of monocyte-derived immature DC into mature DC both morphologically and phenotypically. Intriguingly, when aAPC were forced to attach via a newly identified surface molecule, PladX, and ROCK activity was subsequently blocked, K562-derived aAPC “differentiated” into DC-like cells by acquiring dendrite extensions and growth cone-like structures at the end of the extensions (see picture). PladX-mediated strong attachment was critical for differentiation, since ROCK inhibition without attachment or following attachment via conventional adhesion molecules such as poly-L lysine, fibronectin, or collagen was not sufficient to induce dendrites. Confocal microscopy analysis revealed that dendrites were composed of F-actin rich filopodia and lamellipodia. Furthermore, F-actin and microtubules were differentially localized in the “growth cones” and “dendrite shafts” of aDC, respectively. While treatment with actin inhibitors blocked the generation of “growth cones” but not dendritic shafts, exposure to microtubule inhibitors abrogated the extension of dendritic shafts. Finally, we were able to demonstrate that aDC were more potent than aAPC in CD8+ T cell stimulatory activity. This was the case despite the fact that differentiation of aAPC into aDC does not alter the expression level of molecularly engineered immunoaccessory molecules MHC class I, CD80, and CD83. The effects of the differentiation on processing and presentation of antigenic peptides were negligible since CD8+ T cell antigen was exogenously pulsed as a fully processed synthetic peptide. Taken together, this result indicates that the dendrite formation and the resultant enlarged surface area are critical determinants of DC's enhanced immunogenicity.
We have succeeded in producing infinite number of aDC with enhanced immunogenicity by differentiating our renewable and standardized K562-based aAPC, which has been already tested in the clinic. This novel aDC may overcome the cumbersome issues inherent to conventional DC and widen the applicability of DC-based immunotherapy for cancer.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal