Abstract
Abstract 2988
Poster Board II-964
In pediatric venous thromboembolism (VTE), duration of hypercoagulability—which has implications for recurrence risk and duration of therapy–is not readily assessed despite a battery of laboratory assays for comprehensive thrombophilia testing. Global clotting assays offer the possibility to better understand duration of hypercoagulability on an individualized basis.
We sought to evaluate overall coagulability and fibrinolytic potential over time in children with acute VTE and to compare these findings with D-dimer and established thrombophilia traits.
The Clot Formation and Lysis (CloFAL) global assay was performed in platelet-poor plasma in 58 children enrolled in a single-institutional prospective inceptional cohort study of VTE at The Children's Hospital, Colorado, between March 2006 and June 2009. This spectrophotometric fibrin registration assay employing clotting activation with dilute tissue factor and phospholipid and fibrinolytic enhancement with tissue plasminogen activator has been previously shown analytically sensitive to physiologic and pathologic alterations in multiple components of the coagulation and fibrinolytic systems (Goldenberg et al., Thromb Res 2005). Hypercoagulability was defined by an area under the curve (AUC) of the CloFAL waveform that exceeded the upper limit of age-appropriate reference values established in healthy children (n=26) using the non-parametric method of Tukey, and hypofibrinolysis was similarly defined as a fibrinolytic index (FI, which relates to the slope of decline in absorbance over the period of 30 minutes following maximal amplitude of clot formation) that was below the lower limit of normal. Coagulation index (CI, measured as the AUC over the first 30 minutes of the assay, indexed to the pooled normal plasma standard) was also evaluated, and compared to established reference ranges. Analyses were grouped by period post-diagnosis, as follows: acute (0-1 month; n=10), subacute (1-3 months; n=12), early chronic (3-6 months; n=10), late chronic (≥1 year; n=32). Comprehensive testing for genetic and acquired thrombophilia states was performed in all subjects, along with serial assessment of D-dimer and automated euglobulin lysis time (ELT).
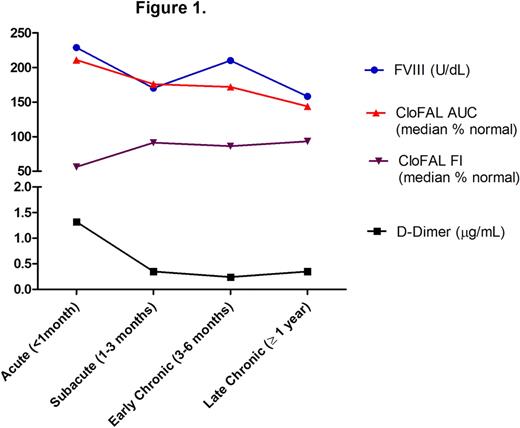
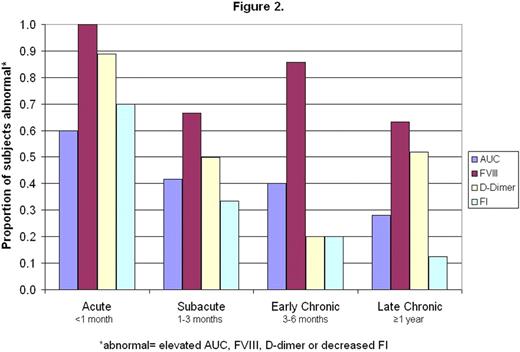
Of the 58 children with VTE evaluated for global coagulative and fibrinolytic capacity, 25% underwent repeat testing in follow-up. A positive relationship with factor VIII activity (FVIII) was demonstrated for both CI and AUC (r=0.37, P=0.006 and r=0.52, P<0.0001). In addition, AUC correlated inversely with FI and directly with ELT (r=-0.58, P<0.0001 and r=0.40, P=0.003), underscoring the interrelatedness between the coagulative and fibrinolytic systems (as has been previously shown, for example, via thrombin-activatable fibrinolytic inhibitor). Figure 1 demonstrates a decreasing trend in values over time post-diagnosis for CloFAL AUC, D-dimer, and FVIII, and increasing trend in CloFAL FI. The proportion of patients with abnormally elevated values for AUC, D-dimer, and FVIII, and abnormally low values for FI, also progressively decreased with time (Figure 2). Notably, however, persistent hypercoagulability was demonstrated in 28% , and hypofibrinolysis in 12.5%, of patients studied ≥1 year post-event (n=32).
These findings demonstrate net hypercoagulability and hypofibrinolysis acutely in the majority of children with VTE, with a trend toward normalization in hemostasis over time across the study population. Further prospective studies are underway to determine whether persistence of hypercoagulability and/or hypofibrinolysis (as observed by global clotting assay in a subset of patients studied here), serves as a prognostic factor for recurrent VTE, and hence may contribute to future risk-stratified approaches to anticoagulant therapy duration.
Off Label Use: The presentation refers to the use of anticoagulants as a drug class in general in the treatment of venous thromboembolism (VTE) in children. Despite their use in the standard care for pediatric VTE, all anticoagulants remain off-label for this indication in children.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal