Abstract
Abstract 2879
Poster Board II-855
Lenalidomide (len) is an immunomodulatory compound with established clinical efficacy and safety in patients with multiple myeloma (MM). Two pivotal phase III trials in patients with relapsed/refractory MM evaluating lenalidomide + dexamethasone (len + dex) vs placebo + dex (MM-009 and MM-010) demonstrated that len + dex is well tolerated with significant improvements in response and overall survival in comparison to placebo + dex (Weber et al, NEJM 2007; Dimopoulos et al, NEJM 2007). Despite a favorable safety profile, neutropenia is one of the most common hematologic adverse events in patients receiving len + dex. Patients who experience neutropenia may require dose adjustments or discontinuation of len + dex therapy. The aim of this study was to understand the incidence, onset, and duration of neutropenia in patients with relapsed/refractory MM receiving long-term administration of len + dex.
Pooled data for patients treated with len + dex from the two phase III studies (MM-009/010) with a median follow-up of 48 months in all patients surviving as of the data cutoff of July 2008, were analyzed for all neutropenia reported as adverse events (neutropenic AE), including all related terms. The change in incidence and severity of neutropenic AE over time was determined by capturing the highest grade of event reported for each patient within each month. Both the severity (grades 1-4) and duration (days) of neutropenic AE were also determined for patients within each month. To evaluate the change in the rate of neutropenic AE for all patients by month, monthly rates were modeled using Poisson regression with a factor for month and the log of the total number of patients included as an offset parameter.
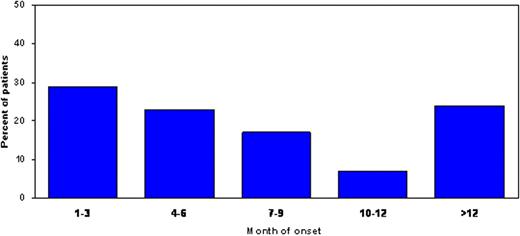
Of 353 patients treated with len + dex, 164 (46%) experienced a neutropenic AE of any grade at some point during the course of the study. A grade 3 or 4 event occurred in 137 (39%) patients. Of the patients who experienced neutropenic AE of any grade, 97 (59%) received G-CSF at some point in the study compared to 8 (4%) of the patients without neutropenic AE. Sixty percent of patients who experienced a neutropenic AE, experienced more than 1 event. Overall, neutropenic AE occurred early, with 52% and 76% of patients experiencing their most severe event within 6 and 12 months of therapy, respectively (Figure). Only 24% of patients who experienced neutropenia experienced their highest grade of event after 12 months of therapy. The median duration of neutropenic AE overall was 10 (25th percentile 8; 75th percentile 29) days. The majority of severe neutropenic AE were grade 3, with very few patients experiencing a grade 4 event. Of the 353 patients treated with len + dex, only 11 (3.1%) patients had febrile neutropenia (12 events). Seven of the patients had their first febrile neutropenia within the first 6 months and 4 patients after 12 months. Twelve patients (3.3%) had their study drug discontinued due to a neutropenic event. Overall, the incidence, severity, and the duration of any neutropenic events did not significantly change over time.
Overall, the first neutropenic AE in patients receiving len + dex occurs early, with more than 50% of patients experiencing their highest grade of event within 6 months of initiation of therapy and only 3.1% of patients treated with len + dex developed febrile neutropenia. These data support the predictable safety profile of len + dex, thereby supporting the long-term use of len + dex in patients with relapsed/refractory MM, and demonstrating the predictable safety profile of this combination therapy.
Occurrence of First Most Severe Neutropenic Event Over Time (n=163)*
* 1 patient missing date of onset
Occurrence of First Most Severe Neutropenic Event Over Time (n=163)*
* 1 patient missing date of onset
Lonial:Gloucester: Research Funding; Novartis: Consultancy; BMS: Consultancy; Millenium: Consultancy, Research Funding; Celgene: Consultancy. Baz:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Swern:Celgene: Employment. Weber:Celgene: Honoraria, Research Funding. Dimopoulos:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal