Abstract
Abstract 2705
Poster Board II-681
Conventional radioimmunotherapy (RIT) with directly radiolabeled anti-B cell antibodies (Ab) induces remissions in 50 to 80% of patients with relapsed or refractory indolent non-Hodgkin lymphomas (NHL). Although administering RIT as consolidation after chemotherapy improves response rates and produces long-term durable remissions in treatment-naïve patients, the β-emitting radionuclides used in current RIT schemes may not be ideal for irradiating the microscopic tumors and the isolated tumor cells present in the setting of minimal residual disease (MRD). RIT with α-emitting radionuclides may be advantageous in the treatment of MRD because the short path length and high energies of α-particles produce optimal cytotoxicity at small target sites while minimizing damage to the surrounding normal tissues. Our group has successfully demonstrated that pretargeted RIT (PRIT) using streptavidin (SA)-Ab and radiolabeled biotin allows rapid specific localization of radioactivity at tumor sites. PRIT using α-emitting radionuclides may be particularly attractive since the most promising α-emitting radionuclides in clinical settings, such as 213Bi (t½ = 46 min), have short half-lives and pretargeting allows the delivery of radioactivity to tumor sites before the activity decays. We therefore performed in vivo studies to evaluate the biodistribution of 213Bi with PRIT. Athymic mice with B-cell NHL (Ramos) xenografts received a tetravalent anti-CD20 (1F5) single-chain (scFv)4SA fusion protein (FP) or a CC49 (scFv)4SA FP (non-binding negative control) followed by an N-acetyl galactosamine clearing agent (CA) and subsequent 213Bi-DOTA-biotin infusion. Tumors, blood, and major organs were collected to determine the percent injected dose per gram (%ID/g) at various time points within ∼3 half-lives of 213Bi. Maximal tumor uptake for 1F5 (scFv)4SA FP was 16.5 ± 7.0 %ID/g at 90 minutes vs. 2.3 ± 0.9 %ID/g for the control FP (p = 0.0001). Biodistributions of 213Bi using a conventional RIT scheme with directly labeled Ab were also evaluated. Athymic mice with Ramos xenografts received 213Bi labeled 1F5 Ab or 213Bi labeled HB8181 Ab (a murine isotype matched nonbinding control). Maximum tumor uptake for 1F5 Ab was 3.0 ± 0.9 %ID/g at 180 minutes vs. 2.3 ± 0.7 %ID/g for the control Ab (p = 0.171). There were no significant differences in tumor uptake and normal organ distribution between the two Ab within 180 minutes of radiolabeled Ab injection presumably due to the protracted circulating half-life of radiolabeled Abs. These results were concordant with our previous experiments using other radionuclides, showing that maximal targeting of radiolabeled Ab occurs between 20 to 24 hours; well beyond the effective half-life of 213Bi. When the results of PRIT and RIT studies were directly compared, tumor-to-blood ratios were 58 to 426-fold higher with PRIT than with conventional RIT. Tumor-to-normal organ ratios of nearly 100:1 were observed with PRIT compared to 3:1 or less with conventional RIT. Using the most favorable PRIT schemes defined in the biodistribution experiments, the therapeutic efficacy of 213Bi was evaluated. Mice treated with PRIT using 1F5 (scFv)4SA FP followed by a CA and 600 μCi 213Bi-DOTA-biotin experienced significant delays in tumor growth. The 1F5 (scFv)4SA FP treated animals had a mean tumor volume of 0.01 ± 0.02 vs. 203.38 ± 83.03 mm3 for the CC49 control group at 19 days (p = 0.0006). The median survival for 1F5 group was not reached after 90 days; whereas, the median survival was 23 days for the CC49 group (p = 0.0019) and 16 days for untreated mice (p = 0.0023). The treatment was well tolerated, with no treatment-related mortalities in any group. These data demonstrate that PRIT using 213Bi has a favorable biodistribution profile and excellent therapeutic efficacy. This model may be particularly effective in MRD settings and further studies are ongoing.
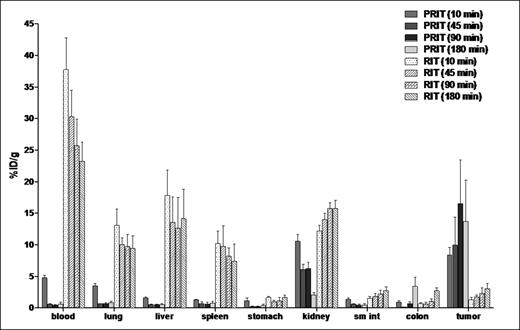
Comparative biodistributions of radioactivity in the tumors, blood, and normal organs of athymic mice bearing Ramos xenografts. The % ID/g of tissue obtained in 2 separate experiments using 1F5 Ab in conventional RIT and PRIT schemes were directly compared.
Comparative biodistributions of radioactivity in the tumors, blood, and normal organs of athymic mice bearing Ramos xenografts. The % ID/g of tissue obtained in 2 separate experiments using 1F5 Ab in conventional RIT and PRIT schemes were directly compared.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal