Abstract
Abstract 2388
Poster Board II-365
The Notch signaling pathway is a critical regulator of cell fate determination and differentiation during development, which is highly cell type specific. Similarly, Notch signaling plays both oncogenic and tumor suppressor roles in a wide variety of malignancies, depending on cell type. In contrast to T cell acute lymphoblastic leukemia (T-ALL) where Notch activation promotes leukemogenesis, induction of Notch signaling in B-ALL leads to growth arrest and apoptosis. The Notch target gene Hairy/Enhancer of Split1 (HES1) is sufficient to reproduce this tumor suppressor phenotype in B-ALL, however the mechanism is not yet known.
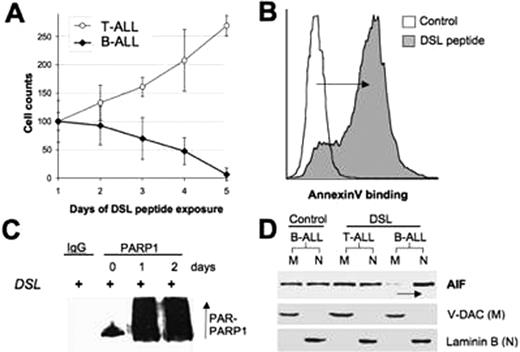
Here we report the novel finding that HES1 forms distinct complexes in B-ALL versus T-ALL. This suggests that HES1 interacting proteins may contribute to the cell-type specific consequences of Notch/HES1 signaling. During characterization of these complexes, we identified the novel interaction between HES1 and PARP1 through immunoprecipitation and MALDI-TOF protein sequencing. This interaction was dependent on the HES1 bHLH and Orange domains and PARP1 and HES1 co-localize to a genomic HES1 binding site by ChIP. This interaction both inhibits HES1 repressor function and induces PARP1 activation in B-ALL. HES1-induced PARP1 cleavage leads to enhanced poly ADP ribosylation of PARP1, consumption of NAD+, diminished ATP levels, and translocation of the Apoptosis Inducing Factor (AIF) from mitochondria to the nucleus, resulting in apoptosis in B-ALL, but not T-ALL. Importantly the potential therapeutic Notch agonist peptide “DSL” also induces cell-specific growth arrest and apoptosis (A+B), followed by poly-ADP ribosylation of PARP1 (C), and nuclear translocation of AIF in B-ALL but not T-ALL cells (D).
These data reveal a novel interaction of HES1 and PARP1 in B-ALL which modulates the function of the HES1 transcriptional complex and signals through PARP1 to induce apoptosis. This novel tumor suppressor mechanism involving a Notch driven, cell-type specific pro-apoptotic pathway may lead to the development of Notch agonist-based cancer therapeutics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal