Abstract
Abstract 1978
Poster Board I-1000
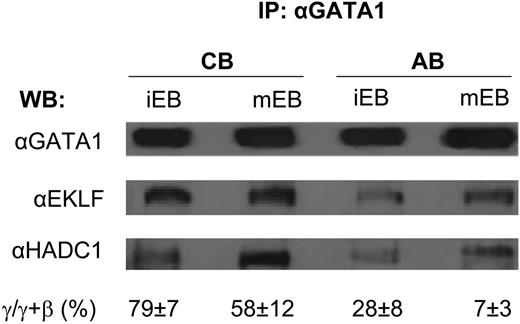
Histone deacetylation is one of the major pathways that maintains chromatin in a condensed configuration preventing gene expression in eukaryotic cells. The deacetylation reaction is catalyzed by the histone deacetylase (HDAC) superfamily, which includes eighteen distinct enzymes. HDACs perform their biological function as multiprotein complexes (Sin3A, NuRD and CoREST) that include at least two HDAC isoforms, DNA docking factors (transcription factors and methyl-binding proteins) and protein kinases (PKC). Data from murine cell lines suggest that association of HDAC1 with EKLF and/or Gata1, which occurs as part of the Sin3A or NuRD complex, may provide specificity to the regulation exerted by this enzyme during erythroid maturation. The role of HDAC complexes in primary human erythroid cells has remained poorly defined. The objective of this study was to characterize HDAC expression in human erythroblasts (EB) and monitor changes in expression and activity during maturation in response to erythropoietin (EPO). Human immature EB (iEB) were generated by culturing adult blood (AB) and cord blood (CB) mononuclear cells for 10-12 days with SCF, IL-3, EPO, dexamethasone and estradiol and then for 24-72 hrs in cultures containing EPO alone (mature EB, mEB) (Migliaccio et al, BCMC 28:168, 2002). The levels of HDAC isoform mRNAs and proteins expressed by iEB and mEB, as well as levels of HDAC1 and HDAC5 activity and association of HDAC1 with either GATA1 or EKLF, were then determined. By quantitative RT-PCR, iEB expressed detectable levels of mRNA for all HDAC isoforms, including SIRT 1 and 2. Induction of maturation had modest effects on the level of HDAC mRNA expressed by the EB with the exception of the mRNA for SIRT2 (increased by 10-fold), HDAC2 and HDAC6 (both increased by 2-3-fold). The increase in HDAC6 mRNA observed with maturation correlated with that of GATA1 (HDAC6 is immediately downstream to GATA1). By western-blot analyses, iEB expressed high levels only of HDAC1 to 5 and SIRT1 and 2. Induction of maturation did not affect the HDAC2 and HDAC3 but decreased HDAC1, HDAC4 and HDAC5 and increased SIRT2 protein levels. Therefore, the levels of mRNAs for these genes remained constant but their protein levels decreased with maturation. To evaluate the effect of decrements in protein level on enzymatic activity, the activity of complexes immunoprecipitated with antibodies specific for HDAC1 and HDAC5, the enzymes whose content decreased the most with maturation, from similar numbers of iEB and mEB was compared. iEB expressed HDAC1 and HDAC5 activity levels 2-fold greater than the standard (HeLa extracts). In agreement with the protein levels, HDAC5 activity decreased (by 1-log) with maturation. However, the activity of HDAC1 increased by 2-fold upon EPO exposure. To further characterize the interactions between transcription factors with HDAC1 within the complex, western-blot analyses of proteins co-immunoprecipitated with GATA1 (or HDAC1) from iEB and mEB obtained from CB and AB were compared (see Figure). A greater fraction of GATA 1 was associated with HDAC1 and EKLF in iEB obtained from CB than in those obtained from AB and in both cases the association increased with maturation. In conclusion, these results extend those previously observed with cell lines (Chen and Bieker, Mol Cell Biol 24:10416, 2004) and suggest that erythroid maturation of primary cells is associated with the dynamic regulation of the HDAC1-complex that includes increased enzymatic activity and ontogenetic-specific re-organization of transcription factors recruited to the complex.
Disclosures: No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2009 by The American Society of Hematology
2009

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal