Abstract
Abstract 1656
Poster Board I-682
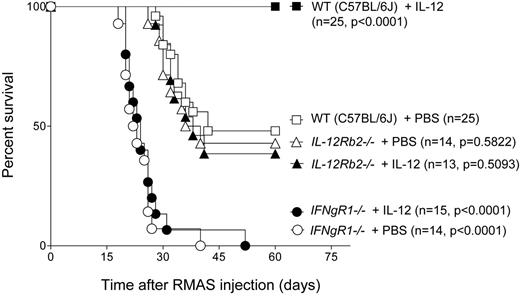
To study the roles of IL-12 and Interferon-gamma (IFNg) in tumor immunity, we used RMAS lymphoma cells to challenge IL-12 receptor beta 2-deficient (IL-12Rb2-/-) and IFNg receptor 1-deficient (IFNgR1-/-) mice that are in the syngeneic C57BL/6J background. We intravenously injected mice with a dose of 1 × 104 RMAS cells that caused death in about 50% of wild-type (WT) mice. As shown in the Figure below, all of the WT mice treated with exogenous IL-12 were rescued from death caused by tumor growth; endogenous IL-12 was not sufficient to impact tumor growth since IL-12Rb2-/- mice showed a survival rate similar to that of WT mice. However, all of the IFNgR1-/- mice succumbed to tumor growth, indicating that endogenous IFNg is required for tumor immunity in this system. Furthermore, IL-12 treatment did not improve the survival of the IFNgR1-/- mice, suggesting that IFNg signaling is required for IL-12's anti-tumor effect.
We previously showed that an IL-12/IFNg axis can inhibit tumor-induced regulatory T cell (Treg) proliferation in vitro (Cao et al, 2008 ASH Annual Meeting). We have subsequently examined their effects on Treg cells in vivo. Compared to naive mice, significant Treg expansion (4.9 ± 2.1 fold, n=5, p=0.025) was observed in the peritoneal cavity of WT mice within 2 weeks after an intraperitoneal injection of 1 × 104 RMAS cells. This expansion was completely blocked by treatment with exogenous IL-12. Treg cells in the IL-12Rb2-/- mice expanded to levels comparable to that in WT animals, suggesting that endogenous IL-12 was not sufficient to control Treg expansion. In contrast, significantly higher Treg expansion was observed in IFNgR1-/- mice (36.8 ± 11.8 fold, n=5, p=0.002), which was partially inhibited by IL-12 treatment (13.2 ± 3.5 fold, n=5, p=0.002), suggesting that an IFNg-independent mechanism may also account for IL-12's anti-Treg effect.
To further study the effects of IL-12 and IFNg on cytotoxic T lymphocyte (CTL) function, we performed mixed lymphocyte reactions (MLR) and used flow-based killing assays (FloKA) to measure cell contact-dependent killing of allogeneic P815 tumor cells. MLR-activated CTLs were found to kill tumor targets via perforin/granzyme-mediated cytotoxicity. At a 10:1 (effector:target) ratio, granzyme AxB-deficient CTLs and perforin-deficient CTLs displayed significantly reduced killing (8.6 ± 1.2% and 4.5 ± 0.9%, respectively) compared to WT CTLs (36.1 ± 3.5%). IL-12 supplement (2ng/ml) to the MLR significantly increased the killing activity of WT CTLs (65.3 ± 4.2%), but had no significant effect on granzyme AxB-deficient CTLs or perforin-deficient CTLs. In contrast, IFNg supplement (10ng/ml) to the MLR had no significant effect on the killing activity of CTLs. Conversely, MLR-activated IFNgR1-/- CTLs killed P815 cells as efficiently as WT CTLs and responded to IL-12 treatment as efficiently as WT CTLs.
Taken together, these data suggest that IL-12 treatment inhibits tumor-induced Treg expansion and stimulates IFNg-dependent anti-tumor immune responses. In addition, IL-12 also activates perforin/granzyme-dependent function of cytotoxic T lymphocytes. These differential effects on diverse immune components may collectively result in enhanced tumor immunity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal