Abstract
Abstract 1472
Poster Board I-495
Platelets are generated from the proplatelets of mature megakaryocyte (MK) in bone marrow (BM). Proplatelet formation (PPF) requires profound changes in the cytoskeletal elements including microtubules and actin fibers. The bending and branching of proplatelet shafts are mediated by actin dynamics (Italiano JE Jr, J Cell Biol, 1999) in the process of PPF. Recent reports showed that Rho and its effecter ROCK, inhibit PPF (Chang Y. et al, Blood, 2007) and that the Wiskott-Aldrich syndrome protein (N-WASP) and its effector Arp2/3 complex, the actin nucleating factor, are essential in PPF (Schulze H. et al Blood, 2006). The contribution of another actin nucleating factor, mammalian diaphanous-related forming (mDia1), which is the other downstream effector of Rho, however, has not been reported in the process of PPF. In this study, we investigated the role of these factors and free barbed ends of actin filaments during PPF using the immunofluorescence method and inhibitory assay with specific inhibitors.
Inhibitory assay: Primary mature MKs, isolated by albumin density gradient method, were cultured in IMDM supplemented with 1% Insulin-Transferin-Selenium (ITS) with the specific signal inhibitor of each signal pathway, including Rho, Rho kinase (ROCK), Rac1 or N-WASP. After incubation at 37°C in 5%CO2 and 20%O2 for 16 hrs, MKs were fixed with 4% paraformaldehyde and then counted the ratio of MKs with PPF. Immunofluorescence method: Isolated MKs were cultured in the same condition, described above, fixed with 4% paraformaldehyde and incubated with the following primary antibodies: anti-mDia1 antibody, anti-Arp3 antibody for 1 hr. After washing, MKs were stained with fluolescence conjugated-antibody against the primary antibody. The free barbed end assay: To allow the visualization of actin nucleation sites, it was performed as described previously (Symons and Mitchison, J Cell Biol, 1991). Fluorescence in MKs was observed under a Zeiss LSM meta confocal microscopy.
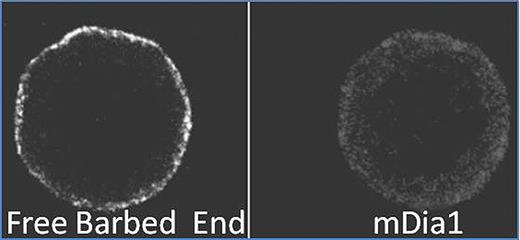
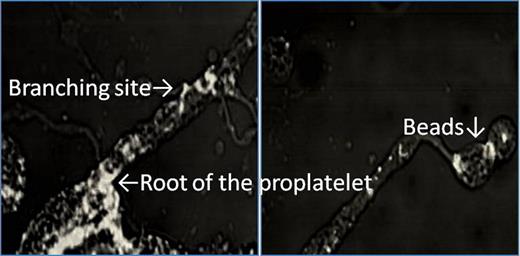
Inhibitory assay: The ratio of MKs with PPF increased significantly at low concentration of cell permeable C3 transferase, the specific inhibitor of Rho, (5 ug/ml)(38.4±7.2%, Control 20.3±8.8%, p<0.05) but decreased significantly at high concentration (data not shown). These data indicated that only ROCK might be inactivated at 5 ug/ml but both ROCK and mDia1 might be inactivated at high concentration. The ratio of MKs with PPF increased in the presence of Y27632, a specific inhibitor of ROCK (10 uM: 51.1±10.2%, Control 20.3±8.8%, p<0.05). However, inhibitor of Rac1 or N-WASP signaling decreased the ratio of MKs with PPF compared to the controls (NSC23766: 50 uM: 8.0±5.2%. Wiskostatin: 50uM: 4±3.4% vs control 18.3±6.8%, p<0.05). These data indicated that Arp2/3 was an important role in PPF, because Rac-1 and N-WASP were located at the upperstream of Arp2/3. Immunofluorescence method & free barbed end assay: Isolated MKs without PPF were round and smooth on their surface, and had a thin F-actin layer under the surface area. Free barbed end (FBE) signals were clearly observed along the plasma membrane and co-localized with mDia1 signals (Figure 1), not with Arp3 signal (data not shown). Thesedata indicated the presence of dynamic equilibrium of actin filaments, regulated by Rho/mDia1 signal, around the plasma membrane of MKs. Considering the previous reports in which Rho/Rock/myosin light chain/myosin IIA pathway was reported to restrain PPF and inhibit releasing premature platelets until the appropriate time, myosin IIA might utilize the actin filaments which was produced via Rho/mDia1 signal. In proplatelets, FBE were accumulated in the roots of proplatelet shaft, bifurcation sites, thin filopodias, and beads (Figure 2). These data indicated that actin filaments, produced at FBE, might have important roles in leading of microtubules at the roots and bifrucation sites and beads formation. mDia1 and Arp3 signals were localized at these sites. The differences of localization between mDia1 and Arp3 signals were that only mDia1 signals were accumulated at thin filopodias around the shaft and bead (data not shown). In conclusion, actin dynamics, which is controlled by the actin nucleating factor Arp3 and mDia1 play important roles in PPF of MKs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal