Abstract
Abstract 1210
Poster Board I-232
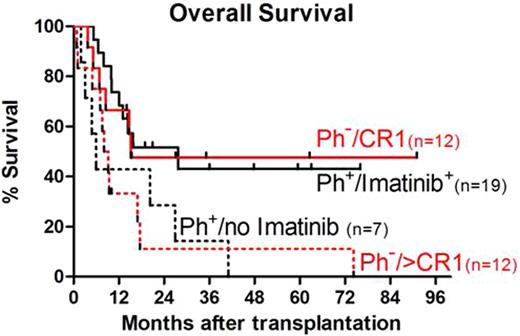
Allogeneic hematopoietic cell transplantation (HCT) with a conventional, high intensity conditioning regimen is an established approach for potentially curing patients (pts) with high-risk acute lymphoblastic leukemia (ALL). However, many pts are not candidates for high intensity conditioning HCT. The use of a reduced intensity conditioning (RIC) for ALL may provide an opportunity to decrease the toxicity of an intense conditioning regimen yet maintain the potential graft vs. leukemia effect. Here we describe the transplantation course and overall survival (OS) of 50 consecutive pts with high-risk ALL that were treated in a multi-center protocol of RIC and allogeneic HCT between February 2000 and August 2008. The median age was 56 (range, 7-69) years. All pts had cytogenetic analysis of bone marrow (BM) at diagnosis. 26 pts had Philadelphia chromosome positive (Ph+) ALL: 18 were in first complete remission (CR1) and 8 were in CR>1 at the time of HCT. 24 pts had Ph–ALL, 12 pts were in CR1 and 12 pts were in CR>1. Pts. were conditioned with fludarabine 90mg/m2 and 2 Gy total body irradiation. Nine pts received peripheral blood stem cells (PBSC) from HLA-identical siblings, 41 pts received grafts from unrelated donors (35 HLA-matched PBSC, 1 BM and 5 received 1-HLA-Ag mismatched PBSC). After the introduction of imatinib mesylate, 19 of the 26 pts with Ph+ALL participated in a study evaluating the safety and efficacy of imatinib starting at day +15 after HCT. Post-grafting immunosuppression consisted of combined mycophenolate mofetil and cyclosporine. Median follow up for surviving pts was 31 months (range, 12-91). One pt had graft rejection (BM recipient). 25 pts (50%) and 4 pts (8%) developed grades 2 and 3-4 acute graft-versus-host disease (GVHD), respectively. Chronic GVHD occurred in 24 pts (53% of pts surviving > 100 days); 15 pts (30%) developed extensive chronic GVHD. 19 pts (38%) relapsed at a median of 4.5 (range, 0.4-59) months. Non relapse mortality (NRM) at 2 and 5 years was 26% and 31%, respectively. Progression-free survival was very similar to OS. For all 50 pts, OS at 2 and 5 years was 37% and 27%, respectively. For pts in CR1 (n=30), OS was 52% and 41%, respectively. In contrast, OS for pts >CR1 (n=20) was significantly lower (13% and 7%, respectively, p=.003). For pts with Ph–ALL, OS was 29%, unchanged at both 2 and 5 years. Those in CR1 had improved OS compared with pts >CR1 (48% vs. 11% unchanged at both 2 and 5 years, respectively, p=0.07, Figure). OS for the 26 pts with Ph+ALL at 2 and 5 years was 45% and 27%, respectively. OS at 2 and 5 years for pts treated with imatinib after HCT was 52% and 43%, respectively, this was superior to the OS for pts with Ph+ALL that did not receive imatinib (p=0.03, Figure). For Ph+ALL pts in CR1 who had no evidence of minimal residual disease (MRD negative, detection by flow cytometry and FISH) prior to HCT and received imatinib (n=12), the OS was 67%, unchanged at both 2 and 5 years. In contrast, the pts with MRD negative Ph+ALL in CR1 who did not receive imatinib (n=4) had OS at 2 and 5 years of 50% and 0%, respectively. Imatinib after HCT was well tolerated: 3 pts required early stopping of imatinib due to associated reversible toxicity. We conclude that RIC with allogeneic HCT is a feasible treatment option that offers durable disease control, particularly for high-risk pts in CR1. The combination of post-transplant imatinib with RIC for Ph+ALL contributed to a substantially improved OS.
Off Label Use: Imatinib - post-grafting treatment. Mycophenolate mofetil - immunosuppression after HCT.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal