Abstract
Acute graft-versus-host disease (aGVHD) often precludes successful immunotherapy of hematologic malignancies with allogeneic T cells. Therefore, we investigated the effect of immunomodulatory superagonistic anti-CD28 monoclonal antibodies (CD28-SA) on the capacity of allogeneic T cells to mediate both aGVHD and the protective graft-versus-tumor (GVT) response. In vivo pretreatment of donor C57BL/6 mice or short-term in vitro culture of donor lymph node cells with a CD28-SA efficiently protected BALB/c recipient mice from aGVHD. This protection strongly relied on the presence of CD28-SA–activated CD4+ CD25+ Foxp3+ regulatory T cells in the donor T-cell inoculum. With respect to the GVT response, CD28-SA–prestimulated T cells were still as potent in clearing lymphoma cells as were T cells without CD28-SA preactivation. Taken together, our data suggest that CD28-SA stimulation of bulk leukocyte cultures in vitro markedly increases the therapeutic window for adoptive immunotherapy with allogeneic T cells in vivo.
Introduction
Apart from acute graft-versus-host disease (aGVHD), severe infectious complications and a relapse of malignant disease threatens patients after allogeneic bone marrow (BM) transplantations.1 Allogeneic T cells contained in the graft cause, on the one hand, aGVHD, but, on the other hand, they also control opportunistic infections, improve engraftment of hematopoietic stem cells, and mediate the graft-versus-tumor (GVT) effect,2 in many instances believed to be a prerequisite for cure from leukemia or lymphoma.1,3 However, standard treatment regimens for aGVHD mainly consist of immunosuppressive drugs, such as cyclosporine A, which not only suppress aGVHD but also mitigate the GVT response and prolong the period of severe immunodeficiency.1
A promising novel approach for the prevention of aGVHD consists of enriching the graft for CD4+ CD25+ Foxp3+ regulatory T cells (Treg cells) before transplantation.4-6 When present in sufficient numbers Treg cells have been shown to deprive conventional T cells (Tconv cells) of cytokines such as interleukin-2 (IL-2),7-9 which induces apoptosis in Tconv cells7 and enhances the production of anti-inflammatory cytokines such as IL-10 by the Treg cells.9,10 Moreover, interaction of CD152 (CTLA-4) expressed by Treg cells with B7 molecules expressed by Tconv cells or antigen-presenting cells is another crucial mechanism by which Treg cells mediate suppression.11-14 With regard to aGVHD, Treg cell–mediated protection from aGVHD is particularly attractive, because it does not abrogate the GVT response.15-17
To achieve protection from aGVHD by Treg-cell enrichment, Treg cells need to be transplanted at approximately a 1:1 ratio with Tconv cells6 ; that is, in relation to Tconv cells, the Treg cells must be enriched by a factor of 5 to 25, depending on species and organ used for isolation of the CD4+ T cells. Therefore, protocols have been developed for in vitro expansion of purified Treg cells.
The protocols used so far rely on a very high purity of the Treg-cell preparation because contaminating Tconv cells grow better than Treg cells upon stimulation with, for example, anti-CD3 and anti-CD28 mAb-coated beads and recombinant IL-2.18 Therefore, these protocols would ideally be substituted by in vitro conditions, which allow for preferential activation and expansion of Treg cells without the need for tedious Treg-cell purification and long-term in vitro culture.
One way for achieving this goal is the use of superagonistic monoclonal anti-CD28 antibodies (CD28-SA) that preferentially activate and expand Treg cells both in vivo and in vitro.19-21 Therefore, we either pretreated donor mice in animal models of aGVHD and GVT responses with a mouse anti–mouse CD28-SA in vivo or added the CD28-SA to total lymph node (LN) cell suspensions in vitro to activate and expand Treg cells before transplantation of purified T cells into allogeneic recipient mice. The latter approach with human peripheral blood mononuclear cells as starting material may bear clinical potential because it avoids the in vivo use of anti–human CD28-SA, for which there is at present no safe protocol.22
Methods
Animals
CD90.1-congenic C57BL/6 mice were bred at the animal facility of the Institute for Virology and Immunobiology, University of Würzburg. C57BL/6.OlaHsd BM donors and BALB/c.OlaHsd hosts for aGVHD experiments were obtained from Harlan-Winkelmann. C57BL/6 and C57BL/6.CD90.1-congenic mice were used for experiments between 6 and 12 weeks of age. BALB/c mice were irradiated at the age of 9 to 10 weeks. All experiments were performed according to the Bavarian state regulations for animal experimentation and approved by the Regierung von Unterfranken as the responsible authority.
Fluorescence-activated cell sorting
The following monoclonal antibodies were used: anti-CD4 Alexa Fluor 647, anti-CD8 PE-Cy5, anti-CD25 FITC (fluorescein isothiocyanate) or biotin, anti-CD90.1 PE (phycoerythrin) or biotin, anti-CD90.2 biotin, anti-B220 Alexa Fluor 647, anti–I-Ab biotin, anti-IFNγ PE, annexin V PE (all BD PharMingen), anti-Foxp3 PE or PE-Cy5 (eBioscience), unconjugated anti-BCL1 idiotype (clone Mc106A5), dαrat Ig PE (Dianova).
Stainings were performed with up to 106 cells in 50 μL phosphate-buffered saline (PBS)/0.1% bovine serum albumin/0.02% NaN3. FcγRII/III receptors were blocked by incubation with saturating amounts of cell culture supernatant of the clone 2.4G2. Fluorochrome-conjugated or biotinylated mAbs were added after blocking (15 minutes, 4°C). Bound biotinylated antibodies were detected by incubation with either PE-Cy5 or allophycocyanin-conjugated streptavidin (BD PharMingen). The cells were analyzed on a FACSCalibur flow cytometer with the use of Cell Quest software (all Becton Dickinson). For further analyses of the data, either Cell Quest or FlowJo (TreeStar Inc) software was used. Dot plots and histograms are shown as log10 fluorescence intensities on a 4-decade scale.
For intracellular staining of Foxp3, cells labeled with antibodies at the cell surface were fixed for 30 minutes at room temperature with fixation buffer (eBioscience) before permeabilization (permeabilization buffer; eBioscience). The cells were blocked with rat serum before staining with anti-Foxp3 mAb for 30 minutes at room temperature. To determine IFNγ expression, splenocytes were first restimulated with phorbol myristate acetate/ionomycin (5/500 ng/mL; Sigma-Aldrich Chemie GmbH) for 4 hours in the presence of GolgiPlug (1:1000; BD) during the last 2 hours of culture. Cells were then stained intracellularly for IFNγ and Foxp3 expression in parallel.
aGVHD experiments and tracking of allo–T-cell responses in vivo
BALB/c mice were conditioned for transplantation by total body irradiation with 8 Gy as a single dose. The mice were assigned to the different experimental groups so that the average body weight before irradiation was about the same in all groups. To prevent bacterial infections, animals were given Neomycin (250 μg/mL; Sigma-Aldrich Chemie GmbH) and Polymyxin B Sulfate (3 U/mL; Sigma-Aldrich Chemie GmbH) in drinking water, starting 3 days before irradiation until day 28 after transplantation.
Approximately 24 hours after irradiation the mice received 107 T cell–depleted (TCD) BM cells from C57BL/6 mice and 106 CD4+ or 8 × 105 CD4+ CD25− peripheral T cells from CD90.1-congenic C57BL/6 mice intravenously. To obtain TCD BM cells, erythrocytes were lysed from total BM preparations by incubation with TAC buffer (20 mM Tris, 155 mM NH4Cl, pH 7.2, 10 minutes, room temperature), then FcRs were blocked with 20 μg/mL normal mouse Ig (Sigma-Aldrich Chemie GmbH) before T cells were depleted using magnetic-activated cell sorting anti-CD90.2 beads (Miltenyi Biotec Inc) and magnetic-activated cell sorting separation columns according to the manufacturer's instructions. T-cell depletion was approximately 95% on average. CD4+ T cells were purified from erythrocyte-lysed splenocytes with average purities of 90% by negative magnetic selection of cells expressing CD8α, CD11b, B220, CD49b, and/or Ter-119 (Miltenyi Biotec Inc). CD25+ cells were depleted either by directly adding a biotinylated anti-CD25 antibody to the other lineage markers or by staining CD4+ cells with anti-CD25 FITC followed by anti-FITC beads (Miltenyi Biotec Inc).
For in vivo preactivation of Treg cells, the superagonistic mouse/anti–mouse CD28 mAb D66523 (mIgG1, endotoxin < 0.01 EU/μg; Serotec) was applied at a dose of 250 μg/mouse in 250 μL PBS intraperitoneally 3 days before isolation of splenic CD4+ cells. Control treatment consisted of either 250 μg/mouse MOPC-31C (mIgG1) or PBS only.
To test for the suppressive activity of Treg cells from PBS- or CD28-SA–treated donors in vivo, pooled spleen/LN cell suspensions were used to magnetically purify CD4+ CD25+ Treg cells (80% Foxp3+).
In vitro preactivation was performed using total LN cell suspensions of C57BL/6.CD90.1-congenic mice cultured at 2 × 106 cells/mL in RPMI 1640 medium (PAA) supplemented with 10% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, nonessential amino acids, 100 U/mL penicillin and 100 μg/mL streptomycin, 30 μM mercaptoethanol, and 2 mM l-glutamine (all Invitrogen) with the D665 mAb added in solution (40 μg/mL). In parallel cultures LN cells depleted of CD25+ cells were mixed with CD4+ CD25+ LN cells from C57BL/6 mice to substitute for the depleted Treg cells. After 4 days in culture, CD4+ T cells were isolated using magnetic separation columns. When added, C57BL/6 Treg cells were depleted by magnetic labeling of CD90.2 Treg cells (66% depletion of Foxp3+ cells). As further controls CD4+ cells were also isolated from freshly prepared LN cells.
In short-term in vivo experiments CD90.1+ CD4+ T cells were labeled with CFSE (carboxyfluorescein succinimidyl ester diacetate; 2.5 or 5 μM; MoBiTec) and 4 or 7 × 106 CD4+ or CD4+ CD25− T cells were transferred intravenously. Absolute numbers of donor CD4+ Foxp3− T cells in secondary lymphoid organs and liver 3 and 6 days after transplantation were calculated by multiplying the absolute cell numbers per organ with the percentages of cells as determined by fluorescence-activated cell sorting analysis.
Observers blinded to the treatment measured body weight and scored clinical appearance of the animals every other day as follows (scores in parentheses)24 : weight loss was less than 10% (0), greater than 10% to less than 25% (1), or greater than 25% (2); posture was normal (0), hunching noted only at rest (1), or severe hunching impairs movement (2); activity was normal (0), mildly to moderately decreased (1), or stationary unless stimulated (2); fur texture was normal (0), mild-to-moderate ruffling (1), or severe ruffling/poor grooming (2); skin integrity was normal (0), scaling of paws/tail (1), or obvious areas of denuded skin (2). At each observation time values were added to obtain 1 cumulative clinical scoring value per animal. Mice with less than 70% of the initial body weight for more than 2 days were humanely killed. Alternatively, animals were killed independently of their body weight to prevent severe suffering as indicated by their overall clinical appearance.
BCL1 lymphoma model
Irradiated BALB/c mice were injected with 3 × 103 freshly thawed BCL1 lymphoma cells 4 hours before transfer of TCD BM cells and, when applicable, also magnetically purified whole T cells from spleen or LNs (92% purity on average). For in vivo Treg-cell activation, donor C57BL/6.CD90.1-congenic mice received 250 μg D665 in 250 μL PBS intraperitoneally 3 days before isolation of the T cells from total splenocytes. In vitro, T cells were isolated from cell suspensions prepared from LNs of C57BL/6.CD90.1-congenic mice and cultured for 4 days in the presence of D665 (80 μg/mL). Whenever possible, splenocytes of humanely killed animals were analyzed for the prevalence of BCL1 cells by staining with the anti-BCL1 idiotype mAb Mc106A5. The lymphoma was rated as “end stage” when both greater than 75% of all splenocytes stained positive with Mc106A5 mAb and the number of BCL1 cells in the spleen exceeded 107.
In vivo killing assay
Six days after BCL1, TCD BM and, when applicable, T-cell transplantation, recipient BALB/c mice were inoculated with a mixture of CFSE-labeled indicator cells: 2 × 106 BCL1 cells, 106 freshly purified BALB/c B cells, and 106 freshly purified C57BL/6 B cells. Fresh C57BL/6 and BALB/c B cells were obtained by negative magnetic depletion of pooled splenocytes and LN cells from CD90.2+ cells (≥ 85% B220+ CD3−). Sixteen hours after the indicator cell transfer, the spleens of recipient mice were analyzed for the prevalence of all 3 subsets of indicator cells by counterstaining for BCL1 idiotype and I-Ab expression and gating on CFSE+ cells. In addition, absolute cell numbers of the progeny of the initially transplanted T cells isolated from either PBS- or D665-treated animals were calculated, based on the total number of splenocytes retrieved and the frequencies of CD90.1+ CD8+ and CD90.1+ CD8− cells as determined by fluorescence-activated cell sorting analysis.
Histology
Formalin-fixed small and large bowel sections (5 μm) were stained with hematoxylin and eosin, and an observer blinded to the prior treatment evaluated the histopathologic changes (adapted from Hill et al25 and Cooke et al26 ) for the small bowel of lamina propria lymphocytic infiltrate, villous blunting, luminal sloughing of cellular debris, and outright crypt destruction, and for the large bowel of lamina propria lymphocytic infiltrate, mucosal ulceration, and outright crypt destruction. The severity of pathologic changes was graded as follows (scores in parentheses): normal (0), focal and rare (0.5), focal and mild (1), diffuse and mild (2), diffuse and moderate (3), or diffuse and severe (4). Scores were cumulated into a single value per animal.
Statistics
Summary graphs were generated with Excel 11.3.5 (Microsoft), and P values are the results of 2-tailed Student t tests, assuming equal variance within groups except for the statistical testing of long-term survival, whereby a χ2 test (GraphPad Prism 4.0c; GraphPad Software Inc) was used. Repeated-measures ANOVA testing (GraphPad Prism 4.0c) was used as indicated. P values less than .05 were considered statistically significant.
Results
Polyclonal Treg-cell activation protects from aGVHD
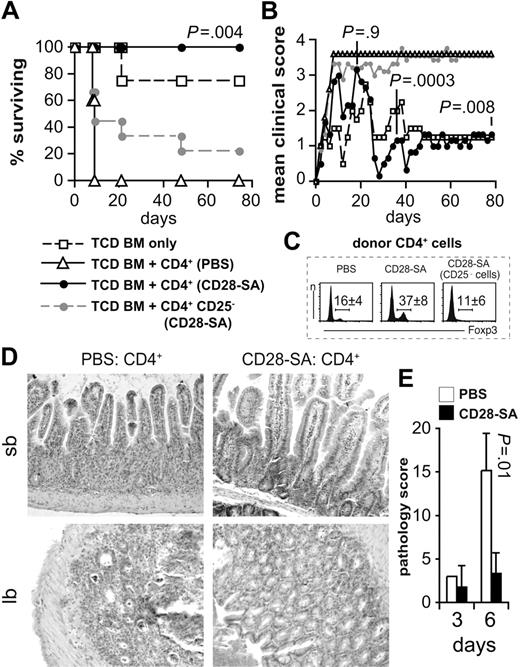
To address whether polyclonal activation of donor T cells with a CD28-SA would reduce the severity of aGVHD, we transplanted TCD BM cells from C57BL/6 mice and CD4+ T cells from CD90.1-congenic C57BL/6 donor mice treated either with a mouse anti–mouse CD28-SA or with PBS into lethally irradiated BALB/c recipient mice. Although all recipients of CD4+ T cells from PBS-treated donors had to be humanely killed by day 9 (Figure 1A), recipients of CD4+ T cells from CD28-SA pretreated donors only transiently underwent aGVHD (Figure 1B) and survived until the end of the observation period (Figure 1A). Depletion of CD25+, that is, Foxp3+ Treg cells, before transplantation (Figure 1C) rendered CD4+ T cells of CD28-SA pretreated mice almost as pathogenic as those of PBS-treated donors (Figure 1A-B). Histologic sections of samples from the aGVHD target organs, small and large bowels, taken on day 6 after T-cell transplantation showed that the protection from clinical signs of aGVHD by CD28-SA pretreatment of donor mice was paralleled by reduced lymphocytic infiltrates into the lamina propria accompanied by less destruction of the gut architecture (Figure 1D-E). Therefore, polyclonal preactivation of donor T cells by a CD28-SA in vivo efficiently protected T-cell recipients from aGVHD both clinically and with respect to aGVHD-associated histopathologic changes.
Polyclonally activated Treg cells protect from aGVHD. Lethally irradiated BALB/c mice were reconstituted with 107 C57BL/6 TCD BM cells either alone (n = 4) or together with 106 CD4+ cells from PBS-treated mice (n = 5) or from CD28-SA–treated donors (250 μg/mouse; n = 6) or 8 × 105 CD4+ CD25− cells from CD28-SA–treated mice (n = 9). (A) The percentages of animals surviving over time and (B) the mean clinical scores of recipient animals are depicted. P values refer to the comparison of recipients of CD4+ versus CD4+ CD25− cells from CD28-SA–treated donors. The experiment has been repeated twice with similar result. (C) Increased frequencies of Foxp3+ cells among donor CD4+ cells 3 days after CD28-SA treatment compared with PBS-treated mice. The numbers indicate mean percentages ± SD of 5 independent analyses. (D) Representative hematoxylin and eosin stainings of small bowel (sb) and large bowel (lb) sections obtained from mice 6 days after transplantation show reduced aGVHD-associated pathology in recipients of CD4+ T cells from CD28-SA–treated donors. Original magnification ×200 with LEICA DMIRE2 microscope, N Plan L 20×/0.40 objective lens, LEICA DFC300 FX camera, and LEICA IM50 Image Manager as the acquisition software. Adobe Photoshop CS3 was used to adjust for brightness and contrast. (E) Histopathologic changes were scored on small and large bowel sections as detailed in “Histology.” Cumulative scores are depicted as means ± SD of 3 mice per group (CD28-SA, day 3; n = 2).
Polyclonally activated Treg cells protect from aGVHD. Lethally irradiated BALB/c mice were reconstituted with 107 C57BL/6 TCD BM cells either alone (n = 4) or together with 106 CD4+ cells from PBS-treated mice (n = 5) or from CD28-SA–treated donors (250 μg/mouse; n = 6) or 8 × 105 CD4+ CD25− cells from CD28-SA–treated mice (n = 9). (A) The percentages of animals surviving over time and (B) the mean clinical scores of recipient animals are depicted. P values refer to the comparison of recipients of CD4+ versus CD4+ CD25− cells from CD28-SA–treated donors. The experiment has been repeated twice with similar result. (C) Increased frequencies of Foxp3+ cells among donor CD4+ cells 3 days after CD28-SA treatment compared with PBS-treated mice. The numbers indicate mean percentages ± SD of 5 independent analyses. (D) Representative hematoxylin and eosin stainings of small bowel (sb) and large bowel (lb) sections obtained from mice 6 days after transplantation show reduced aGVHD-associated pathology in recipients of CD4+ T cells from CD28-SA–treated donors. Original magnification ×200 with LEICA DMIRE2 microscope, N Plan L 20×/0.40 objective lens, LEICA DFC300 FX camera, and LEICA IM50 Image Manager as the acquisition software. Adobe Photoshop CS3 was used to adjust for brightness and contrast. (E) Histopathologic changes were scored on small and large bowel sections as detailed in “Histology.” Cumulative scores are depicted as means ± SD of 3 mice per group (CD28-SA, day 3; n = 2).
Secondary expansion of polyclonally activated Treg cells after transfer into the allogeneic host maintains a beneficial Treg cell/Tconv cell ratio
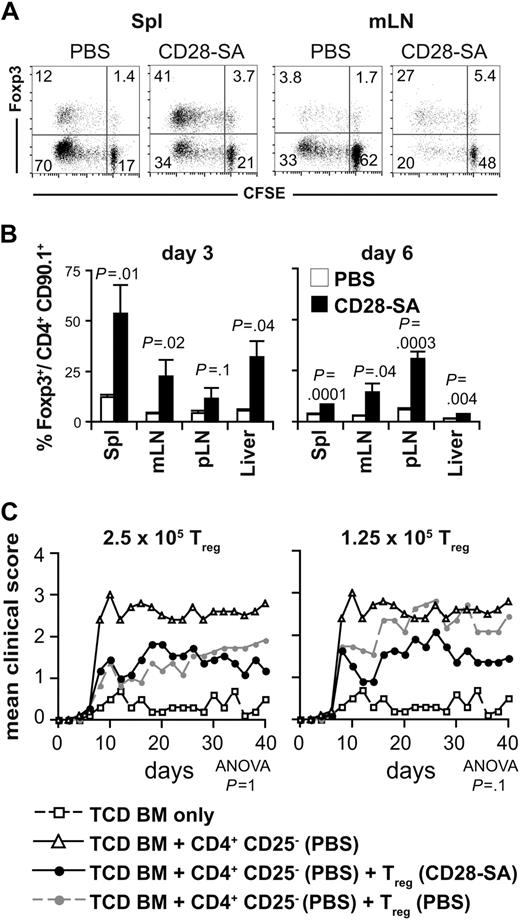
Although the preactivation and expansion of donor T cells by CD28-SA treatment in vivo greatly increases the ratio of Treg cells to Tconv cells (Figure 1C), the degree of proliferation of Treg cells versus Tconv cells after transplantation into the allogeneic host determines whether this beneficial bias toward the Treg cells will be maintained. Therefore, we analyzed the proliferative history of donor Treg cells and Tconv cells labeled with the fluorescent dye CFSE 6 days after transfer into BALB/c recipients. CD28-SA–preactivated Treg cells strongly proliferated after transfer into allogeneic hosts as did Treg cells from PBS-treated mice, both indicated by CFSE dye dilution among Foxp3+ donor CD4+ T cells (Figure 2A). Despite preactivation, the Treg cells, however, did not suppress the proliferation of Foxp3− conventional donor T cells (Figure 2A). Still, Treg-cell frequencies remained increased among CD4+ T cells from CD28-SA– versus PBS-treated donors re-isolated from the spleens, LNs and the livers, another aGVHD target organ, of recipient mice on days 3 and 6 after transplantation (Figure 2B) and even on day 78 (N.B. and T.K., unpublished data, September 2006).
Secondary expansion of polyclonally activated Treg cells after transfer into the allogeneic host sustains a beneficial Treg cell/Tconv cell ratio. (A) CFSE dilution among CD4+ CD90.1+ Foxp3+ and Foxp3− donor T cells isolated from spleen (Spl) or mesenteric LNs (mLN) 3 days after transplantation into lethally irradiated BALB/c hosts. The figures represent the percentages of cells per quadrant. (B) Frequencies of Foxp3+ cells among CD4+ CD90.1+ donor T cells 3 and 6 days after transplantation. The bars indicate means ± SD of 3 mice per group. pLN indicates peripheral LNs. The data are representative for 5 experiments with similar results. (C) Direct comparison of suppressive activity of Treg cells from PBS- or CD28-SA–treated donors in vivo. Either 2.5 (n = 11) or 1.25 × 105 CD4+ CD25+ Treg cells (n = 11) from PBS- or CD28-SA–treated mice were transplanted into lethally irradiated BALB/c recipients together with 2.5 × 105 CD4+ CD25− cells from mice treated with PBS and 107 C57BL/6 TCD BM cells. Control mice received only TCD BM cells (n = 10) or TCD BM cells together with CD4+ CD25− cells (PBS; n = 10). Data from 2 experiments were pooled. P values refer to the comparison of recipients of Treg cells from either PBS- or CD28-SA–treated donors.
Secondary expansion of polyclonally activated Treg cells after transfer into the allogeneic host sustains a beneficial Treg cell/Tconv cell ratio. (A) CFSE dilution among CD4+ CD90.1+ Foxp3+ and Foxp3− donor T cells isolated from spleen (Spl) or mesenteric LNs (mLN) 3 days after transplantation into lethally irradiated BALB/c hosts. The figures represent the percentages of cells per quadrant. (B) Frequencies of Foxp3+ cells among CD4+ CD90.1+ donor T cells 3 and 6 days after transplantation. The bars indicate means ± SD of 3 mice per group. pLN indicates peripheral LNs. The data are representative for 5 experiments with similar results. (C) Direct comparison of suppressive activity of Treg cells from PBS- or CD28-SA–treated donors in vivo. Either 2.5 (n = 11) or 1.25 × 105 CD4+ CD25+ Treg cells (n = 11) from PBS- or CD28-SA–treated mice were transplanted into lethally irradiated BALB/c recipients together with 2.5 × 105 CD4+ CD25− cells from mice treated with PBS and 107 C57BL/6 TCD BM cells. Control mice received only TCD BM cells (n = 10) or TCD BM cells together with CD4+ CD25− cells (PBS; n = 10). Data from 2 experiments were pooled. P values refer to the comparison of recipients of Treg cells from either PBS- or CD28-SA–treated donors.
To determine whether Treg cells from CD28-SA–treated donors were not only higher in frequencies but also more suppressive on a per cell basis than Treg cells from PBS-treated mice, we cotransferred purified Treg cells from PBS- or CD28-SA–injected mice together with CD4+ CD25− T cells from PBS-treated mice into irradiated BALB/c recipients (Figure 2C). In contrast to other in vivo models27 and in vitro results,19,21,27 Treg cells from CD28-SA–injected donors were only in tendency more potent in mediating protection from aGVHD in vivo than Treg cells from PBS-treated mice (Figure 2C).
CD28-SA treatment of donor mice, thus, did not increase the suppressive activity of Treg cells for aGVHD, but the vivid secondary expansion of Treg cells after transfer to the allogeneic host maintained a balanced ratio of Treg cells to Tconv cells.
Reduced accumulation and delayed differentiation of allogeneic Foxp3− CD4+ cells in the presence of CD28-SA–activated Treg cells
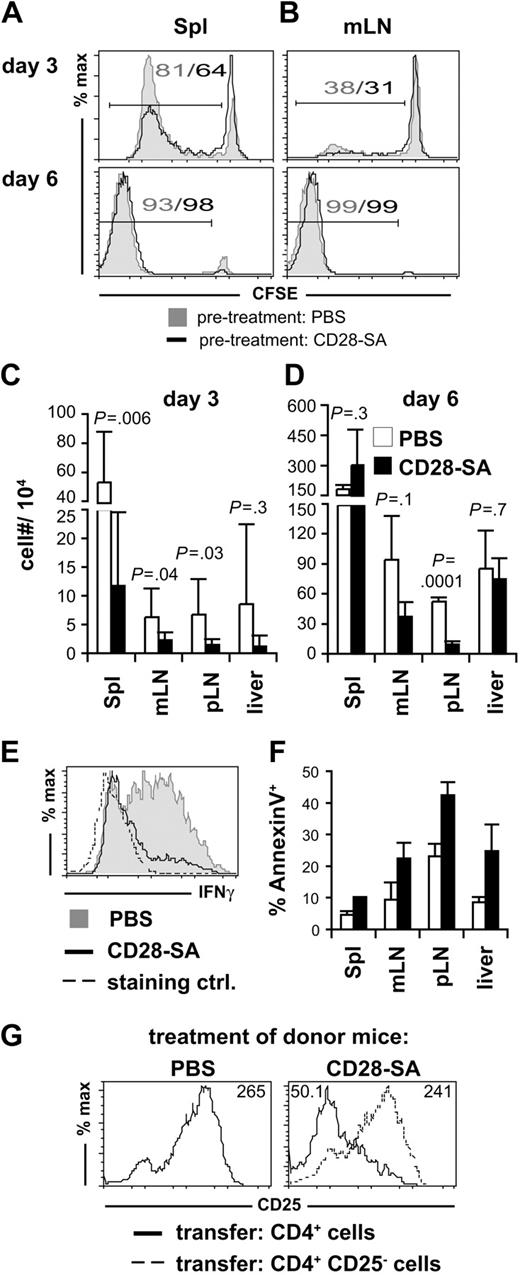
Although CD28-SA–preactivated Treg cells did not suppress the proliferative response of pathogenic Tconv cells (Figure 2A), a closer analysis of the CFSE dye dilution profiles 3 days after transplantation indicated that fewer Tconv cells had entered the cell cycle or that the cycled cells did not accumulate as well as in the absence of CD28-SA preactivation (Figure 3A-B). In line with this observation we recovered lower absolute numbers of donor CD4+ Tconv cells from recipient mice 3 days (Figure 3C) and, at least in LNs, also 6 days after transplantation (Figure 3D) when we had treated the donor mice with the CD28-SA compared with PBS. Apart from the accumulation of Tconv cells, CD28-SA treatment also interfered with the differentiation of Tconv cells to IFNγ-producing effector T cells (Figure 3E).
Reduced accumulation and differentiation of pathogenic CD4+ cells in the presence of CD28-SA–activated Treg cells. CFSE dye dilution among CD4+ CD90.1+ Foxp3− donor T cells recovered from (A) spleen (Spl) or (B) mesenteric LNs (mLN) 3 and 6 days after transplantation into lethally irradiated BALB/c mice. Numbers indicate the frequencies of CFSElow cells. Absolute numbers of donor CD4+ Foxp3− T cells in Spl, mLN, peripheral LNs (pLN), and liver 3 (C) and 6 (D) days after transplantation. Data from 3 independent experiments were pooled, and bars represent means ± SD of 3 to 9 mice per group. (E) IFNγ expression by CD4+ CD90.1+ Foxp3− cells isolated from mLN of BALB/c mice 6 days after transplantation. As a control cells from PBS-treated donors were stained for IFNγ expression without restimulation in vitro. (F) The percentages of annexin V+ cells among CD4+ CD90.1+ Foxp3− donor T cells were determined on day 6 after transplantation (PBS, n = 3; mLN, n = 2). (G) Polyclonally activated Treg cells suppress CD25 expression by Foxp3− donor CD4+ cells. Cells were isolated from spleens 4 days after transplantation. Numbers indicate mean fluorescence intensities.
Reduced accumulation and differentiation of pathogenic CD4+ cells in the presence of CD28-SA–activated Treg cells. CFSE dye dilution among CD4+ CD90.1+ Foxp3− donor T cells recovered from (A) spleen (Spl) or (B) mesenteric LNs (mLN) 3 and 6 days after transplantation into lethally irradiated BALB/c mice. Numbers indicate the frequencies of CFSElow cells. Absolute numbers of donor CD4+ Foxp3− T cells in Spl, mLN, peripheral LNs (pLN), and liver 3 (C) and 6 (D) days after transplantation. Data from 3 independent experiments were pooled, and bars represent means ± SD of 3 to 9 mice per group. (E) IFNγ expression by CD4+ CD90.1+ Foxp3− cells isolated from mLN of BALB/c mice 6 days after transplantation. As a control cells from PBS-treated donors were stained for IFNγ expression without restimulation in vitro. (F) The percentages of annexin V+ cells among CD4+ CD90.1+ Foxp3− donor T cells were determined on day 6 after transplantation (PBS, n = 3; mLN, n = 2). (G) Polyclonally activated Treg cells suppress CD25 expression by Foxp3− donor CD4+ cells. Cells were isolated from spleens 4 days after transplantation. Numbers indicate mean fluorescence intensities.
A reduced accumulation of donor Tconv cells in the presence of preactivated Treg cells might be a consequence of increased apoptosis because of Treg cell–mediated withdrawal of cytokines such as IL-2 from proliferating Tconv cells.7 Indeed, we detected a higher frequency of annexin V+ cells among postmitotic Tconv cells in the presence of CD28-SA–activated Treg cells versus nonactivated Treg cells (Figure 3F). Because IL-2 induces the expression of CD25, the α chain of its high-affinity receptor, in an autocrine loop, reduced CD25 expression by Tconv cells in the presence of CD28-SA–preactivated Treg cells (Figure 3G) suggested that cytokine withdrawal might at least in part also contribute to the immunomodulatory activity of preactivated Treg cells. Thus, CD28-SA stimulation of donor T cells mediated protection from aGVHD by inhibiting both the accumulation and the effector cell differentiation of conventional donor CD4+ T cells.
T cells maintain antilymphoma activity after in vivo pretreatment of donor mice with a CD28-SA
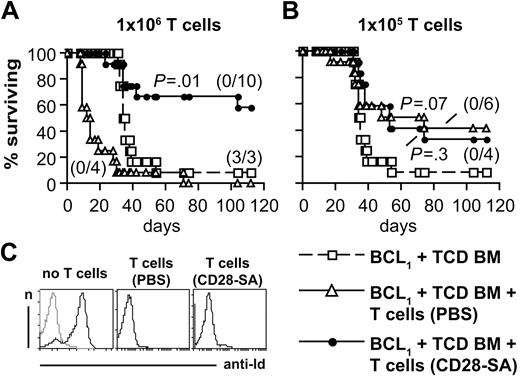
Long-term survival of patients with hematologic malignancies receiving an allogeneic BM transplant strongly depends on the eradication of residual tumor cells because of the GVT effect mediated by allogeneic T cells contained in the graft. Therefore, we inoculated BALB/c mice with BCL1 lymphoma cells28 followed by therapeutic transplantations of BM cells together with total, that is, CD4+ and CD8+, T cells from either PBS- or CD28-SA–treated donor mice to see whether T cells from CD28-SA–treated mice were capable of mounting a curative GVT response.
When we transplanted 106 T cells from CD28-SA–treated mice, 58% of recipient mice survived long term (Figure 4A) with none of the mice analyzed showing a high prevalence of idiotype-positive BCL1 lymphoma cells in postmortem analyses of splenocytes (Figure 4C). In contrast, recipients of whole T cells from PBS-treated donors more rapidly died of aGVHD than did the animals treated with TCD BM cells only who developed end-stage BCL1 lymphoma approximately 3 weeks later (Figure 4A,C). Ten-fold less T cells from PBS-treated control mice did not induce premature death from aGVHD and still protected the mice from the BCL1 lymphoma, allowing for long-term survival of 42% of the recipient BALB/c mice (Figure 4B). As with the high dose, T cells from CD28-SA–treated donors also did not induce premature death from aGVHD when the low dose of cells was transferred and still mediated some degree of protection form the BCL1 lymphoma, leading to long-term survival of 33% of the mice compared with 8% in the group treated solely with TCD BM cells (Figure 4B).
T cells maintain antilymphoma activity after in vivo pretreatment of donor mice with a CD28-SA. (A) Survival of lethally irradiated BALB/c mice inoculated with BCL1 lymphoma cells 4 hours before TCD BM cell transfer. 106 T cells (A) or 105 T cells (B) from donor mice treated as indicated were transferred together in 1 experiment with the TCD BM cells and in the other 4 days later. Both experiments rendered similar results. Therefore, data from the 2 experiments were pooled (n = 12). Differences between mice receiving donor T cells and the TCD BM only group were subjected to statistical testing. Numbers in parentheses indicate animals with end-stage lymphoma/animals analyzed. (C) The histograms show the detection of BCL1 idiotype-positive cells (black) in the spleens of mice from the indicated groups. A control staining without the anti-idiotypic antibody is depicted in gray.
T cells maintain antilymphoma activity after in vivo pretreatment of donor mice with a CD28-SA. (A) Survival of lethally irradiated BALB/c mice inoculated with BCL1 lymphoma cells 4 hours before TCD BM cell transfer. 106 T cells (A) or 105 T cells (B) from donor mice treated as indicated were transferred together in 1 experiment with the TCD BM cells and in the other 4 days later. Both experiments rendered similar results. Therefore, data from the 2 experiments were pooled (n = 12). Differences between mice receiving donor T cells and the TCD BM only group were subjected to statistical testing. Numbers in parentheses indicate animals with end-stage lymphoma/animals analyzed. (C) The histograms show the detection of BCL1 idiotype-positive cells (black) in the spleens of mice from the indicated groups. A control staining without the anti-idiotypic antibody is depicted in gray.
In comparison to mice receiving the high dose of CD28-SA–activated T cells, protection from aGVHD was not enhanced in animals treated with the low T-cell dose (Figure 4B). This seeming paradox might be explained by a delayed killing of BCL1 cells in mice receiving 105 T cells compared with mice treated with 106 T cells. This delayed killing, in turn, might have added further momentum to aGVHD because BCL1 cells, like endogenous antigen-presenting cells,29 also carry T cell–activating alloantigens.
Polyclonal activation of T cells in vivo, thus, greatly increased the therapeutic window for successful immunotherapy of the BCL1 lymphoma with allogeneic T cells by reducing aGVHD severity, while maintaining anti-BCL1 lymphoma activity.
Specific killing of alloantigen-expressing target cells by T cells from CD28-SA–treated donors
The long-term survival of BCL1 lymphoma–bearing BALB/c mice receiving T cells from CD28-SA–treated donors suggested that a specific immune response against alloantigen–expressing cells, including the BCL1 cells, had arisen in these animals. To assess whether this was, indeed, the case we injected CFSE-labeled alloantigen–expressing BCL1 and freshly isolated BALB/c B cells, as well as alloantigen–nonexpressing C57BL/6 B cells into BALB/c recipient mice 6 days after transplantation of C57BL/6 T cells (Figure 5A). As for the long-term experiments we also used mice that had only been treated with TCD BM cells to control for the effects of the donor T cells.
T cells from CD28-SA–treated donors specifically kill alloantigen-expressing target cells with similar efficiency as do T cells from PBS-treated donors. (A) Experimental design. FACS indicates fluorescence-activated cell sorting. (B) Representative dot plots used for identifying the different indicator cell populations at the end of the in vivo killing assay are shown. (C) Circles represent individual animals and the horizontal bars the median values with 5 animals per group (T cells [PBS], n = 4). (D) The spleens of mice that had been transferred with T cells from either PBS- or CD28-SA–treated donor mice harbored similar numbers of donor CD90.1+ CD8− and CD90.1+ CD8+ T cells at the time of the analysis of the killing assay. The experiment has been repeated with in vitro CD28-SA–activated T cells rendering similar results.
T cells from CD28-SA–treated donors specifically kill alloantigen-expressing target cells with similar efficiency as do T cells from PBS-treated donors. (A) Experimental design. FACS indicates fluorescence-activated cell sorting. (B) Representative dot plots used for identifying the different indicator cell populations at the end of the in vivo killing assay are shown. (C) Circles represent individual animals and the horizontal bars the median values with 5 animals per group (T cells [PBS], n = 4). (D) The spleens of mice that had been transferred with T cells from either PBS- or CD28-SA–treated donor mice harbored similar numbers of donor CD90.1+ CD8− and CD90.1+ CD8+ T cells at the time of the analysis of the killing assay. The experiment has been repeated with in vitro CD28-SA–activated T cells rendering similar results.
We observed a similar degree of killing of alloantigen–expressing target cells by T cells from either PBS- or CD28-SA–treated donors (Figure 5B-C). In comparison to mice without T-cell transplantation, the odds ratios of BCL1 cells and freshly isolated BALB/c B cells, both carrying the alloantigen H-2d, to freshly isolated alloantigen-negative C57BL/6 B cells, were reduced to a similar degree in the presence of T cells from either PBS- or CD28-SA–treated donors (Figure 5B-C). This similar degree of killing was achieved without a significant difference in the absolute numbers of donor-derived CD8− and CD8+ T cells from both groups (Figure 5D), indicating that on a per cell basis T cells from PBS- and CD28-SA–treated donors carried equal cytotoxic potential. This is in line with the notion that it was the alloantigen-specific immune response, which allowed for elimination of the BCL1 lymphoma and long-term survival of animals transplanted with T cells from CD28-SA–treated donors.
Short-term in vitro activation of allogeneic T cells with a CD28-SA protects from aGVHD while maintaining GVT activity
Although in vivo applications of superagonistic anti-CD28 mAbs into both rats and mice are very well tolerated, there is of yet no safe protocol for polyclonal Treg-cell activation with a CD28-SA in humans. Therefore, we attempted to substitute the in vivo treatment of donor mice for polyclonal Treg-cell activation by performing in vitro stimulations of unseparated LN cells from C57BL/6.CD90.1-congenic mice with the anti–mouse CD28-SA.
Four days of culture in the presence of the CD28-SA increased the frequency of Foxp3+ cells from 14% among freshly isolated LN CD4+ cells to 41% among cultured CD4+ cells (Figure 6A). With respect to absolute numbers, Treg cells were increased by approximately 40%, whereas Tconv cells were decreased by approximately 60% (N.B. and T.K., unpublished data, December 2007).
Short-term activation of allogeneic T cells with a CD28-SA in vitro protects from aGVHD while maintaining GVT activity. (A) Increased frequencies and (B) activated phenotype of Foxp3+ Treg cells after CD28-SA stimulation of whole LN cells in vitro. Numbers represent the percentages of Foxp3+ cells as indicated by the marker. (C) Lethally irradiated BALB/c mice were reconstituted with 107 C57BL/6 TCD BM cells either alone or together with 5 × 105 CD4+ or 4 × 105 Treg cell–depleted CD4+ cells (n = 10) characterized in panel A. P values compare (C) survival curves or (D) clinical scores from animals after transfer of cultured CD4+ cells versus Treg cell–depleted CD4+ cells. Data from 2 individual experiments were pooled. BALB/c recipient mice received BCL1 lymphoma cells followed by TCD BM cells only or TCD BM cells together with either (E) 5 × 105 or (F) 5 × 104 fresh T cells or the same numbers of T cells isolated from whole LN cell cultures stimulated in vitro with a CD28-SA for 4 days. Data from 2 experiments were pooled (n = 12; 5 × 104 T cells [fresh], n = 11), and P values were obtained comparing T-cell recipients with the TCD BM only group. Numbers in parentheses indicate animals with end-stage lymphoma/animals analyzed).
Short-term activation of allogeneic T cells with a CD28-SA in vitro protects from aGVHD while maintaining GVT activity. (A) Increased frequencies and (B) activated phenotype of Foxp3+ Treg cells after CD28-SA stimulation of whole LN cells in vitro. Numbers represent the percentages of Foxp3+ cells as indicated by the marker. (C) Lethally irradiated BALB/c mice were reconstituted with 107 C57BL/6 TCD BM cells either alone or together with 5 × 105 CD4+ or 4 × 105 Treg cell–depleted CD4+ cells (n = 10) characterized in panel A. P values compare (C) survival curves or (D) clinical scores from animals after transfer of cultured CD4+ cells versus Treg cell–depleted CD4+ cells. Data from 2 individual experiments were pooled. BALB/c recipient mice received BCL1 lymphoma cells followed by TCD BM cells only or TCD BM cells together with either (E) 5 × 105 or (F) 5 × 104 fresh T cells or the same numbers of T cells isolated from whole LN cell cultures stimulated in vitro with a CD28-SA for 4 days. Data from 2 experiments were pooled (n = 12; 5 × 104 T cells [fresh], n = 11), and P values were obtained comparing T-cell recipients with the TCD BM only group. Numbers in parentheses indicate animals with end-stage lymphoma/animals analyzed).
After CD28-SA stimulation, the Treg cells had an activated phenotype as indicated by increased expression of both Foxp3 (Figure 6A) and CD25 in comparison to freshly isolated cells (Figure 6B). Transfer of CD4+ T cells purified from these LN cell cultures compared with freshly isolated CD4+ T cells protected BALB/c recipient mice from aGVHD-associated lethality and clinical signs of the disease (Figure 6C-D).
Upon in vitro stimulation with a CD28-SA, a significant subpopulation of Tconv cells also expresses CD25,20 which is not the case after in vivo treatment.27 To be able to directly assess the role of in vitro–expanded Treg cells in the protection from aGVHD in vivo, we substituted the CD90.1+ donor Treg cells with CD90.2+-congenic Treg cells before starting the LN cell culture. This allowed us to deplete the Treg cells before transplantation via the CD90.2 marker and, thus, independently of CD25 expression. Moreover, the congenic marking of the Treg cells showed that CD28-SA stimulation in vitro expanded preexisting Treg cells as among Treg cell–depleted CD4+ cells only 14% were Foxp3+ versus 41% among nondepleted CD4+ cells (Figure 6A).
As for in vivo CD28-SA applications, depletion of Treg cells before transplantation abolished most of the protective effect of CD28-SA prestimulation (Figure 6C-D). Therefore, the preferential activation and expansion of Foxp3+ Treg cells over conventional T cells during in vitro culture with a CD28-SA translated into Treg cell–mediated protection from aGVHD in vivo.
Finally, we used T cells after CD28-SA stimulation in vitro for adoptive therapy of mice bearing the BCL1 lymphoma. Forty-two percent of mice receiving 5 × 105 CD28-SA–stimulated T cells survived until the end of the observation period at day 122 (Figure 6E), whereas mice treated with TCD BM cells only succumbed to the BCL1 lymphoma with a median survival time of 28 days and no long-term survivors (Figure 6E). Recipient mice treated with 5 × 105 freshly isolated T cells developed terminal aGVHD 18 days earlier than the mice that received a transplant with TCD BM cells only (Figure 6E). Similar to the results obtained after CD28-SA stimulation in vivo (Figure 4C), lowering the number of transplanted T cells by 10-fold allowed for long-term survival of 17% of mice from both groups (Figure 6F). Because 2 of 7 mice and 5 of 8 mice, respectively, developed end-stage BCL1 lymphoma after transfer of 5 × 104 T cells (Figure 6F), we estimate that this T-cell number was already close to the lower limit for a curative antilymphoma response to occur.
Taken together, polyclonal activation of T cells by CD28-SA treatment of donor mice in vivo as well CD28-SA stimulation of unseparated LN cells in vitro greatly widened the therapeutic window for antilymphoma therapy with allogeneic T cells by preserving the GVT effect, on the one hand, and protecting mice from aGVHD, on the other hand.
Discussion
Immunotherapy with allogeneic T cells bears great potential not only for hematologic malignancies but also for solid tumors.30 Allogeneic T-cell transplantation will, however, only be more widely applicable if aGVHD can be sufficiently controlled. Therefore, novel approaches like the one reported here with the use of a CD28-SA for polyclonal activation of Treg cells leading to efficacious protection from aGVHD in a mouse model are necessary for further clinical development of allogeneic T-cell therapy.
With respect to such clinical protocols for patients it was crucial that we could substitute the in vivo treatment of donor mice by in vitro culture of unseparated LN cells in the presence of the CD28-SA because the only clinically tested anti–human CD28-SA TGN1412 had caused a toxic cytokine storm during a first-in-man study.22 Although the amount of cytokines released was much more pronounced after TGN1412 administration than after the treatment of mice or rats with a CD28-SA, data from a human/mouse xenograft model indicate that superagonistic anti–human CD28 stimulation can mediate preferential expansion of human Treg cells over Tconv cells in vivo31 and, possibly, also in vitro.
In contrast to the cytokine storm observed in humans, CD28-SA–mediated preactivation of allogeneic T cells, either in vivo or in vitro, was highly efficient in preventing aGVHD in mice, which strongly depended on the presence of CD28-SA–activated CD4+ CD25+ Foxp3+ donor Treg cells in the recipient animals. The preferential expansion of Treg cells further allowed us to leave the Treg cells untouched and, therefore, prevent loss of suppressive activity during Treg-cell purification. This appears to be all the more important because CD28-SA preactivation did not significantly increase the suppressive activity of Treg cells on a per cell basis. We hypothesize that the strong activation of Treg cells after transplantation into allogeneic hosts levels the differences in activation status of CD28-SA–stimulated versus “resting” Treg cells that are readily detectable in vitro19,21,27 and in other disease models in vivo.27
Protection from aGVHD after CD28-SA stimulation was, however, not only mediated by higher frequencies of Treg cells in the donor cell inoculum and/or a higher susceptibility of Tconv cells to Treg cell–mediated suppression because we still observed some degree of protection from aGVHD when we had depleted CD25+ cells before transplantation. This reduced capacity of CD28-SA–stimulated Tconv cells to induce aGVHD might be due to the suppressive effects exerted by Treg cells on the Tconv cells during the prestimulation period with the CD28-SA in vivo21 and/or due to the preactivation of the Tconv cells as such. In line with the latter hypothesis, CD28-SA stimulation increased the frequencies of effector/memory-like CD62low cells among CD4+ Foxp3− T cells (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), a phenotype associated with a reduced capacity to induce aGVHD.32 In line with both hypotheses, Tconv cells also showed a reduced propensity to secrete a proinflammatory mix of cytokines in vitro after CD28-SA treatment in vivo (supplemental Figure 1).
Not quite unlike CD28-SA stimulation, adding rapamycin to anti-CD3/anti-CD28–stimulated T cells in vitro also increases the frequency of Foxp3+ Treg cells among CD4+ T cells, provided the cells receive a sufficient costimulatory signal via CD28.33 In vivo protection from aGVHD by rapamycin in a mouse model,34,35 however, also abrogated the desired GVT response against a leukemia cell line.35 Here, superagonistic anti-CD28 stimulation may help to improve the GVT activity of Tconv cells cultured in the presence of rapamycin by enhancing their survival36 because CD28-SA stimulation is superior to conventional costimulation in protecting T cells from apoptotic death.37
Contrary to the data that have been obtained with rapamycin treatment so far, T cells preactivated with a CD28-SA either in vivo or in vitro triggered a curative GVT response in vivo. Transient aGVHD, which correlated with a break-through expansion of Tconv cells in the spleens, but not LNs, of these animals might be a prerequisite for successful lymphoma eradication as it, indeed, seems to be for patients.1,3 Lowering the number of non-preactivated T cells in our model also endowed recipient mice with a survival advantage in comparison to mice without T-cell therapy. This mimics today's clinical practice whereby some patients are, indeed, cured of lymphoma or leukemia by allogeneic BM transplantations. However, because clinicians have to decide on how many T cells to transplant without actually knowing the patient's individual risk of developing severe aGVHD, any protocol reducing the incidence and severity of aGVHD at a given T-cell number, while not abrogating the GVT response, constitutes a major therapeutic advantage.
In summary, CD28-SA preactivation of allogeneic T cells facilitates immunotherapy of hematologic malignancies by reducing aGVHD severity and, at the same time, maintaining GVT activity of the allogeneic T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Karen Balbach, Yvonne Gold, Nadine Pfeifer, and Sandra Werner for conducting experiments and providing excellent technical assistance, and Christian Linden for helping with BM preparations. We thank Martin J. Glennie for providing us with the BCL1 lymphoma cells and the anti-idiotypic mAb Mc106A5.
This work was supported by the Wilhelm Sander-Stiftung (grants 2005.133.1 and 2005.133.2).
Authorship
Contribution: N.B. designed and performed research, performed statistical analyses, and wrote the manuscript; X.D. performed research and proofread the manuscript; T.H. initiated the study, designed research, and edited the manuscript; and T.K. designed research and sketched and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Kerkau, Universität Würzburg, Institut für Virologie und Immunbiologie, Versbacherstr 7, D-97078 Würzburg, Deutschland; e-mail: kerkau@mail.uni-wuerzburg.de.

![Figure 5. T cells from CD28-SA–treated donors specifically kill alloantigen-expressing target cells with similar efficiency as do T cells from PBS-treated donors. (A) Experimental design. FACS indicates fluorescence-activated cell sorting. (B) Representative dot plots used for identifying the different indicator cell populations at the end of the in vivo killing assay are shown. (C) Circles represent individual animals and the horizontal bars the median values with 5 animals per group (T cells [PBS], n = 4). (D) The spleens of mice that had been transferred with T cells from either PBS- or CD28-SA–treated donor mice harbored similar numbers of donor CD90.1+ CD8− and CD90.1+ CD8+ T cells at the time of the analysis of the killing assay. The experiment has been repeated with in vitro CD28-SA–activated T cells rendering similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/20/10.1182_blood-2009-04-218248/4/m_zh89990943940005.jpeg?Expires=1769102580&Signature=1Rq6-uXqXZJFfuLJ6KXi44XOet0csa59UvjpqJ80V884qGpplD990b5wrvGWegNr7vZO8pcrFLKiB0j7ZAUVpBdGXn4Zt4Py-2oT12KrWDXUbmKKUHg7qntvt-aQLpNca~~avsYU1rIvJtIovdFFaG~mNIvbw~iqy67DVlJfQ2GtwM8hzHorJ2d2RZ4FmUogylFYOgb913n8s3CL6CuPx7-crJKT7D-fX0CsKv3kWbdRey7kxxHG~oFnnyQ76C9HV9CnP7aEEgTljpOX3nQh1-4pm2x0L4NzZepnHYBilwwJZFfQ7FzRtnEJu0qHtJxRfnF3Uk71SOi2h7g0-gEJyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Short-term activation of allogeneic T cells with a CD28-SA in vitro protects from aGVHD while maintaining GVT activity. (A) Increased frequencies and (B) activated phenotype of Foxp3+ Treg cells after CD28-SA stimulation of whole LN cells in vitro. Numbers represent the percentages of Foxp3+ cells as indicated by the marker. (C) Lethally irradiated BALB/c mice were reconstituted with 107 C57BL/6 TCD BM cells either alone or together with 5 × 105 CD4+ or 4 × 105 Treg cell–depleted CD4+ cells (n = 10) characterized in panel A. P values compare (C) survival curves or (D) clinical scores from animals after transfer of cultured CD4+ cells versus Treg cell–depleted CD4+ cells. Data from 2 individual experiments were pooled. BALB/c recipient mice received BCL1 lymphoma cells followed by TCD BM cells only or TCD BM cells together with either (E) 5 × 105 or (F) 5 × 104 fresh T cells or the same numbers of T cells isolated from whole LN cell cultures stimulated in vitro with a CD28-SA for 4 days. Data from 2 experiments were pooled (n = 12; 5 × 104 T cells [fresh], n = 11), and P values were obtained comparing T-cell recipients with the TCD BM only group. Numbers in parentheses indicate animals with end-stage lymphoma/animals analyzed).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/20/10.1182_blood-2009-04-218248/4/m_zh89990943940006.jpeg?Expires=1769102580&Signature=UhlAmmUfIx0xquaWetCv6QehZgPAlnNq7a22aJVjc8yUE2N2ntTxexdFYFXosLOITVhirOgUJQNgK~hm4vQ~j3oZUoUFrPJmOrMxus7RMC6p6n0HG4cBW~6C9xv66jXtiPHNJLTIbE1xS3fGdl4VJSVz92vIZ7qfQ9O0Qu0qCTmcu9yLLkOm-QPBTv2UeZLKv~PUeswH3NjUSvph8GNsHDZ3djnqCevo1YkGfbJMSAc88aauHS3s8kzBK5DR52vV84sluoeaF5tN80b1-7qPRoWXQNEfhItm3wr45e3WBdTFomN8sirHL3Tfv999wIwCGSFiOQ51u4GOlh2VwaJFMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal