Abstract
The liver is the current site for pancreatic islet transplantation, but has many drawbacks due to immunologic and nonimmunologic factors. We asked whether pancreatic islets could be engrafted in the bone marrow (BM), an easily accessible and widely distributed transplant site that may lack the limitations seen in the liver. Syngeneic islets engrafted efficiently in the BM of C57BL/6 mice rendered diabetic by streptozocin treatment. For more than 1 year after transplantation, these animals showed parameters of glucose metabolism that were similar to those of nondiabetic mice. Islets in BM had a higher probability to reach euglycemia than islets in liver (2.4-fold increase, P = .02), showed a compact morphology with a conserved ratio between α and β cells, and affected bone structure only very marginally. Islets in BM did not compromise hematopoietic activity, even when it was strongly induced in response to a BM aplasia-inducing infection with lymphocytic choriomeningitis virus. In conclusion, BM is an attractive and safe alternative site for pancreatic islet transplantation. The results of our study open a research line with potentially significant clinical impact, not only for the treatment of diabetes, but also for other diseases amenable to treatment with cellular transplantation.
Introduction
The liver was suggested as an optimal site for islet transplantation by Lacy and colleagues, using a rat model of diabetes.1 By the 1980s, successful transplantation of islet autografts was reported in humans by using infusion of cells into the patient's liver through the portal venous circulation.2-4 Subsequently, the publication of the first case of insulin independence in a diabetic patient after infusion of islets through the portal vein consecrated the liver as the site of choice for the islet transplantation in humans.5 The subsequent clinical experience of islet transplantation has been developed almost exclusively using the intrahepatic infusion through the portal vein.6
In the last years, however, it has becoming increasingly recognized that the liver may not be the optimal environment as a recipient site for pancreatic islets, owing not only to immunologic7,8 but also anatomic9,10 and physiologic factors that most likely contribute to the decline of islet mass after implantation.11-13 Intrahepatic islet infusion in humans is associated with an immediate blood-mediated inflammatory reaction, thrombosis, and hepatic tissue ischemia with elevated blood liver enzymes.12,14-19 Loss of as many as 50% to 75% of islets during engraftment in the liver has been suggested to be a prime factor necessitating the very large number of islets needed to achieve normoglycemia. Furthermore, the necessity for cannulation of the portal system to seed the islets produces an increase in portal pressure proportional to the islet mass administered by infusion,20 thus restricting the total mass that can be implanted. As a consequence, a highly purified suspension of islets is needed to transplant sufficient cells to achieve insulin independence. Because the purity of the suspension is inversely proportional to the islet yield per donor,21 fewer islets can be isolated from the already scarce donor pool, further limiting broad clinical applicability of pancreatic islet transplantation. The recognition of these problems has renewed the interest in the search for an alternative site for implantation, such as the intramuscular site and the omental pouch.22
Bone marrow (BM) may represent an ideal alternative site for pancreatic islet transplantation, thanks to its protected and extravascular (but well-vascularized) microenvironment.23,24 Because of its broad distribution and easy access, BM has the potential to overcome not only the physiologic loss of islets, but also the technical limitations and complications encountered with the intraportal infusion.20 To address the potential of BM as an alternative site for pancreatic islet transplantation, we implanted syngeneic pancreatic islet isografts (C57BL/6 islets to C57BL/6 mice) into BM of diabetic recipients and assessed short- and long-term graft survival, function, and safety in comparison with the liver site. The results show that the BM is a more suitable site than the liver for the implantation of islets in this model.
Methods
Islet isolation and culture
Pancreatic islets were isolated from C57BL/6 or BALB/c mice (9 weeks old, 20-22 g; Charles River Laboratories) by a collagenase digestion method. Briefly, 2 mL of cold Hanks buffer/collagenase type V solution (1 mg/mL; Sigma-Aldrich) was infused into the pancreatic duct in situ, and the removed pancreas was digested at 37°C for 15 minutes. Islets were purified on a discontinuous Ficoll gradient (Sigma-Aldrich). The islets (250 islets/mL) were cultured freely floating (37°C, 5% CO2) in medium RPMI 1640 (BioWhittaker) supplemented with l-glutamine (Sigma-Aldrich), penicillin-streptomycin (1000 U/mL to 10 mg/mL; Sigma-Aldrich), and 10% (vol/vol) fetal calf serum (HyClone) for 20 to 24 hours before the transplantation. Islet purity was more than 90%.
Mice
Male C57BL/6 mice (9 weeks old, 20-22 g; from Charles River Laboratories) were used as recipients. Mice were made diabetic (nonfasting blood glucose levels between 400 and 600 mg/dL) with intravenous streptozocin (STZ) injection (175 to 200 mg/kg; Sigma-Aldrich) 1 week before transplantation. Blood glucose measurements were performed using a Glucometer Elite (Bayer). The animals had free access to tap water and pelleted food throughout the course of the study. The animal ethics committee of San Raffaele Scientific Institute approved all experiments.
Islet transplantation
Islets were transplanted via the portal vein, as previously described.25 Islets were transplanted in the BM as follows: recipients were anesthetized with isoflurane, a 0.5-cm longitudinal incision was made in front of the right knee, and the plate of the femur was exposed and trepanized with a 3/32-inch shank carbide burr in the direction of the medullar channel. Islets packed in PE-50 polyethylene tubing (BD Biosciences) were then introduced into the medullar channel. When the tubing was withdrawn, the cluster of islets was left in the medullar channel by the vacuum effect. The skin was closed with 4-0 silk.
Evaluation of graft function
Blood sugar levels were measured 15, 30, and 60 minutes after the end of the surgical procedure, daily for the first week, and then every second day after transplantation. Surgical death was defined as death within the first 7 days after transplantation. Euglycemia was defined as nonfasting blood glucose levels less than 200 mg/dL for 2 consecutive measurements after islet transplantation. An intravenous glucose tolerance test (IVGTT) and an oral glucose tolerance test (OGTT) were performed at 1, 3, 6, and 12 months after transplantation to evaluate the function of the grafted islets. IVGTT was initiated after a 16-hour fast; mice were given glucose (0.5 g/kg) by tail vein injection. Blood samples were obtained at 0, 1, 5, 15, 20, 30, and 60 minutes after injection and were used to determine glucose concentrations. From the IVGTT, the glucose tolerance was quantified from the glucose elimination constant (KG; expressed as percentage of elimination of glucose per minute) as the reduction in circulating glucose between 1 and 15 minutes (KG1-15) after intravenous administration, following logarithmic transformation of the individual plasma glucose values.26 A similar estimation was performed for the total 1- to 60-minute glucose disappearance rate (KG1-60). This parameter indicates the rate of glucose disappearance during the whole test, when the delayed insulin effect is properly accounted for. OGTT was initiated after a 4-hour fast; mice were given glucose (1 g/kg) by oral gavage. Blood samples were obtained at 0, 10, 20, 30, 60, 90, and 120 minutes after glucose administration and were used to determine glucose concentrations. The area under the curve (AUC) for glucose during OGTT was calculated using the trapezoidal method (baseline = 0 minutes). Serum insulin and glucagon were determined by enzyme-linked immunosorbent multiple assay (Mouse Endocrine Lincoplex Kit; Linco Research). The minimal modeling of insulin and glucose data from 4-hour fasting blood sample was used to assess the β-cell function. Homeostatic model assessment (HOMA) provided equations for estimating β-cell function and insulin resistance27 and was estimated using the Oxford HOMA calculator (http://www.dtu.ox.ac.uk).
Evaluation of graft morphology
For morphologic investigations, the recipients were killed 1 year after transplantation. Liver, pancreas, and femur were procured and fixed in 10% buffered formalin and processed routinely for histology. Femurs were placed in Fix Decal (Pro-EKo) overnight to decalcify the bones before embedding in paraffin. Histologic sections were stained with hematoxylin and eosin, anti-insulin guinea pig primary polyclonal antibody (DakoCytomation), anti-glucagon rabbit primary polyclonal antibody (NovoCastra Laboratories), and anti-Ki-67 rabbit primary monoclonal antibody (Thermo Fisher Scientific). The peroxidase-antiperoxidase immunohistochemistry method (PAP Kit; DakoCytomation) or AffiniPure anti–guinea pig tetramethylrhodamine-5 (and 6)–isothiocyanate secondary antibodies (Jackson Immuno Research Laboratories) were used for detection. For quantitative analysis, all immunoreactive cells were analyzed using a Leica DMIRE2 microscope equipped with a color video camera connected to a computer (Hewlett-Packard) and quantified using Axio Vision 4.4 (Carl Zeiss). Islet size was calculated with the image analysis of single measured islets and expressed as area in μm2. For the determination of the fractional β-cell area, each tissue section was imaged at ×40 magnification (×4 objective), and the ratio of the β-cell area:tissue area was digitally quantified. The insulin and glucagon cell numbers were normalized by the total islet cell number of corresponding islets and expressed as its percentage. β-cell size was calculated by dividing insulin-positive areas for the number of the nuclei. Ki-67+/insulin+ cells were reported as percentage of total insulin+ cell. Between 20 (minimum) and 30 islets per case were calculated.
Evaluation of hematopoietic activity of BM
Platelets, white blood cells (WBCs), red blood cells, hemoglobin, and hematocrit values were counted with an automated cell counter (HeCo VetC; SEAC); BM cells were isolated by flushing femurs of treated and control mice. For colony-forming cell (CFC) assay, 2.5 × 104 cells were suspended and grown in Methocult GF M-3434 (StemCell Technologies) in triplicate, and colonies were scored at 10 to 14 days after plating. BM cell phenotyping was carried out by fluorescence-activated cell sorting analysis, after labeling with fluorochrome-conjugated antibodies against B220, CD3, Gr1, Ter119, CD71, c-Kit, Sca1, and Annexin V (BD Pharmingen).
Lymphocytic choriomeningitis virus infection and related procedures
The lymphocytic choriomeningitis virus (LCMV) strain Armstrong28 was used in this study. C57BL/6 mice were infected intravenously with 2 × 105 plaque-forming units of LCMV and weighted daily after infection. Whole blood was collected daily from the retro-orbital plexus, and, in addition to glycemia levels, platelets, WBCs, and hematocrit values were counted with an automated cell counter. Serum LCMV RNA was analyzed by quantitative real-time polymerase chain reaction, as described,28 using the following LCMV-specific primers: 5′-CTCCTTTCCCAAGAGAAGACTAAG-3′ and 5′-TCCATTTGGTCAGGCAATAAC-3′. Single-cell suspensions were prepared from spleens harvested at day 8 postinfection, and intracellular interferon γ (IFN-γ) staining on CD8+ T cells was performed in the presence or absence of the H2b-restricted immunodominant glycoprotein 33 peptide, as previously described.28 Samples were analyzed with a FACSCalibur flow cytometer, and the data were processed using the CellQuest software (BD Immunocytometry Systems).
Evaluation of mouse bone: peripheral quantitative computed tomography analysis
Peripheral quantitative computed tomography (pQCT) measurements were performed using a Stratec Research SA+ pQCT scanner (Stratec Medizintechnik) with a voxel size of 70 μm and a scan speed of 3 mm/second. The graft-bearing femur and the contralateral femur of each mouse were scanned in the horizontal plane using 4 consecutive cross-sectional images at 3.75 mm, 4.25 mm, 5.5 mm, and 6.5 mm proximal to the distal end of the femur. These scanning sections were chosen as representative of graft implant site, and the relevant pQCT parameters included the measurement of the trabecular compartment only at the 2 most distal sections. The scans were analyzed with pQCT software 6.00 using contour mode 2 and peel mode 2 with a threshold of 350 mg/cm3 for the calculation of trabecular and total bone parameters at the metaphysis and with a threshold of 710 mg/cm3 for cortical bone parameters at the diaphysis. The different thresholds of 350 and 710 mg/cm3 for the metaphysis and diaphysis, respectively, were established to account for partial volume effect. The cortical bone density is lower at the metaphysis than at the diaphysis due to the thinner cortex. The threshold was therefore adjusted according to the cortical density to optimize accuracy. The polar strength strain index (SSI) was calculated by the manufacturer's software, as follows: SSI = Σi = 1,n ri2 × aCD/ND × rmax, where “r” is the distance of a voxel from the center of gravity, “rmax” is the maximum distance of a voxel from center of gravity, “a” is the area of a voxel, “CD” is the cortical density, and “ND” is the density of normal cortical bone tissue equal to 1200 mg/cm3, as measured by pQCT when no spaces are included. To account for changes in the mineralization of bone and therefore for changes in material properties, the section modulus was normalized for this value in pQCT software Version 2.7.
Statistical analysis
Data were generally expressed as mean standard error or median (Min value–Max value). Differences between parameters were evaluated using one-way analysis of variance (ANOVA) test, followed by Bonferroni post hoc t test. Multivariate tests of repeated measures ANOVA were used to compare glycemia during the time. The χ2 test was used for categorical variables. Kaplan-Meier analysis was used to compare normoglycemia gain. For all analyses, a 2-tailed P value of .05 was considered significant. Statistical analyses were performed using the Statistical Package for Social Science, Version 13.0 (SPSS).
Results
Islet transplantation in BM
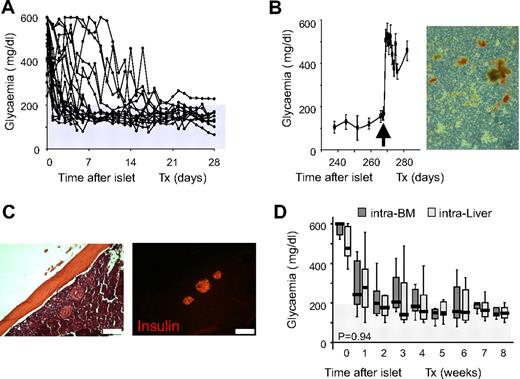
Twenty-four STZ-treated severely diabetic C57BL/6 mice (nonfasting glycemia before transplantation: 509 ± 116 mg/dL) were transplanted with 500 equivalent syngeneic C57BL/6 islets (EI) in BM (Figure 1A). Transplants resulted in normalization of blood glycemia and promptly reverted to hyperglycemia when the graft-bearing BM was removed (Figure 1B). Histologic inspection of femur diaphysis revealed the presence of numerous insulin-immunoreactive cells at the site of implantation (Figure 1C), and dithizone staining of BM in the removed femur showed the presence of insulin-producing islets (Figure 1B). We confirmed these results with a second experiment in which 20 STZ-treated severely diabetic C57BL/6 mice (nonfasting glycemia before transplantation: 519 ± 97 mg/dL) were transplanted with 500 equivalent syngeneic C57BL/6 islets from the same isolation alternatively through the portal vein (intraliver; n = 10) or in BM (intra-BM; n = 10). In both sites, islets displayed functional activity after infusion and glycemia decreased in the first week. Transplants improved metabolic control and gradually reached and maintained maximal function within 3 to 4 weeks (Figure 1D). All in all, these data demonstrate that the islets infused in BM are able to control blood glycemia in vivo.
C57BL/6 syngeneic islet transplantation in BM. (A) Nonfasting glycemia of STZ-treated severely diabetic C57BL/6 mice transplanted with 500 equivalent islets into the BM (n = 24). (B) Left, Nonfasting glycemia of STZ-treated severely diabetic C57BL/6 mice transplanted in BM with 500 equivalent islets. BM of right femur was removed 9 months after transplantation (arrow; n = 6). Values shown are means ± SEM. Right, Dithizone staining of right femur BM contents after removal at 9 months after transplantation. (C) Histologic appearance (×10) of islets in BM 3 months after transplantation. Immunofluorescent images show insulin staining (red). Scale bar 200 μm. (D) Nonfasting glycemia reduction in the 8 weeks after transplantation with 500 equivalent islets according to transplant site. Intraliver: islets infused through the portal vein, n = 10. Intra-BM: islets infused directly into the BM, n = 10. Data are expressed as box plots of mean glycemia of the single weeks. Statistical analysis was performed by tests of repeated measures ANOVA.
C57BL/6 syngeneic islet transplantation in BM. (A) Nonfasting glycemia of STZ-treated severely diabetic C57BL/6 mice transplanted with 500 equivalent islets into the BM (n = 24). (B) Left, Nonfasting glycemia of STZ-treated severely diabetic C57BL/6 mice transplanted in BM with 500 equivalent islets. BM of right femur was removed 9 months after transplantation (arrow; n = 6). Values shown are means ± SEM. Right, Dithizone staining of right femur BM contents after removal at 9 months after transplantation. (C) Histologic appearance (×10) of islets in BM 3 months after transplantation. Immunofluorescent images show insulin staining (red). Scale bar 200 μm. (D) Nonfasting glycemia reduction in the 8 weeks after transplantation with 500 equivalent islets according to transplant site. Intraliver: islets infused through the portal vein, n = 10. Intra-BM: islets infused directly into the BM, n = 10. Data are expressed as box plots of mean glycemia of the single weeks. Statistical analysis was performed by tests of repeated measures ANOVA.
Next, we performed a histologic time-course study of BM after syngeneic islet transplantation (supplemental Figures 1 and 2, available on the Blood website; see the Supplemental Materials link at the top of the online article). Immediately after infusion, islets appeared to be healthy, stained strongly for insulin, and never induced the formation of thrombi. Absence of pathologic changes in islets and surrounding BM tissue was still evident by 24 hours after transplants, a time point in which necrotic areas appear in the central region of islets infused into liver and ischemic necrosis of liver tissue is already evident.25 Note also that BM tissue-remodeling activity was detected at this and later time points exclusively in proximity of the bone hole performed for the islet infusion. After 24 to 72 hours, no evidence of leukocyte infiltration around islets was present (something that occurs in syngeneic livers in response to ischemic necrosis25 ), and the situation remained unchanged until at least day 7.
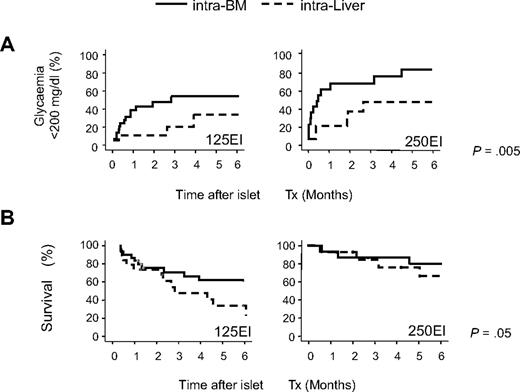
Next, we transplanted smaller numbers of C57BL/6 islets either in the BM or liver of C57BL/6 mice to best compare their functional performance at these sites. Ninety-one STZ-treated severely diabetic mice (mean nonfasting glycemia before transplantation: 540 ± 92 mg/dL) were transplanted with 125 (n = 50, 5120 ± 195 EI/kg) or 250 (n = 41, 11 600 ± 690 EI/kg) equivalent islets alternatively through the portal vein (intraliver: 125 EI n = 20, 250 EI n = 21) or in BM (intra-BM: 125 EI n = 30, 250 EI n = 20). Twelve of 91 mice (13.2%) died during or soon after (within 7 days) the surgical procedure (4 of 50 and 8 of 41, respectively, for intra-BM and intraliver; P = .1, Pearson χ2 test) and were excluded from the subsequent analyses. Islet function significantly differed between the 2 sites (P = .005, log rank statistic adjusted for islet number in Kaplan-Meier analysis; Figure 2A). The probability and the median time to reach euglycemia (< 200 mg/dL) in our marginal islet mass transplantation were as follows: 52% and 85 days for transplants of 125 EI into the BM and 21% and less than 180 days for transplants of 125 EI into the liver (P = .05, log rank test in Kaplan-Meier analysis); 76% and 14 days for transplants of 250 EI into the BM and 43% and 57 days for transplants of 250 EI into the liver (P = .04, log rank statistic in Kaplan-Meier analysis). Multivariate Cox Regression Analysis, including site, islet number, and recipient pretransplant glycemia,29 showed that significant independent factors for reaching euglycemia were as follows: (1) the BM versus liver as recipient site (odds ratio, 2.5; 95% confidence interval, 1.2-5.2; P = .02); (2) the infusion of 250 EI versus 125 EI (odds ratio, 2.2; 95% confidence interval, 1.1-4.3; P = .02); and (3) the pretransplant glycemia (odds ratio, 0.996; 95% confidence interval, 0.993-0.999; P = .01). Finally, mouse survival was significantly higher in mice that received islets in the BM site (P = .05, log rank statistic adjusted for islet number in Kaplan-Meier analysis; Figure 2B).
Comparison of outcomes after islet transplantation into the BM and liver in minimal mass models. C57BL/6 recipients were transplanted with 125 or 250 syngeneic equivalent islets either into the portal vein (intraliver: 125 EI n = 20, 250 EI n = 21) or into the BM (intra-BM: 125 EI n = 31, 250 EI n = 25). Mice that died due to the surgical procedure (4 of 56 and 8 of 41, respectively, for intra-BM and intraliver) were excluded from the analysis. (A) Kaplan-Meier analysis for the gain of normoglycemia (< 200 mg/dL). (B) Kaplan-Meier survival analysis. Differences between the intra-BM and intraliver models were tested using the log rank statistic adjusted for islet number.
Comparison of outcomes after islet transplantation into the BM and liver in minimal mass models. C57BL/6 recipients were transplanted with 125 or 250 syngeneic equivalent islets either into the portal vein (intraliver: 125 EI n = 20, 250 EI n = 21) or into the BM (intra-BM: 125 EI n = 31, 250 EI n = 25). Mice that died due to the surgical procedure (4 of 56 and 8 of 41, respectively, for intra-BM and intraliver) were excluded from the analysis. (A) Kaplan-Meier analysis for the gain of normoglycemia (< 200 mg/dL). (B) Kaplan-Meier survival analysis. Differences between the intra-BM and intraliver models were tested using the log rank statistic adjusted for islet number.
Because impure preparations of islets are often transplanted in humans, we also examined outcome for semipure syngeneic islet grafts in the BM site (n = 19 diabetic mice receiving 200 pure equivalent islets versus 19 diabetic mice receiving 200 equivalent islets mixed with acinar tissue at a 1:1 ratio; supplemental Figure 3). None of the mice died from the surgical procedure. The proportion of mice achieving normoglycemia was similar in both groups, suggesting that the BM site was likely to be suitable also for impure islet preparations.
Graft survival and glucose metabolism after syngeneic islet transplantation in BM and in liver
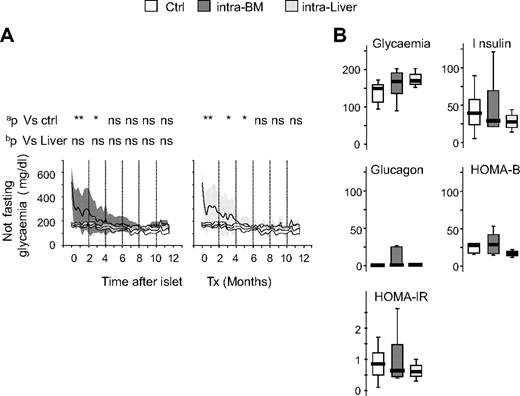
The probability to reach normoglycemia was improved when islets were transplanted in the BM compared with the liver in a minimal mass model. Nevertheless, it is also important that the quality of normoglycemia when reached is at least comparable in both sites. In mice that reached euglycemia (28 of 47 for intra-BM and 10 of 32 for intraliver), islet transplant gradually decreased nonfasting glycemia to normal level within 6 months and maintained function thereafter (1 year) with glucose levels comparable with normal nondiabetic control mice (Figure 3A). In mice that reached euglycemia, glycemia, insulin, glucagon, HOMA-B, and HOMA-IR evaluated after 4 hours of fasting at 6 to 9 months after transplantation were also similar in mice that achieved normoglycemia via islets into BM or islets into liver (Figure 3B). No differences were observed between mice reaching normoglycemia in the intra-BM and intraliver models. Mice that did not reach euglycemia showed glucose levels constantly higher than control, and mice eventually died as a result of uncontrolled diabetes (data not shown).
Graft survival and long-term glucose metabolism after islet transplantation in BM and liver. (A) Blood glucose profile of normal C57BL/6 mice (Ctrl, white; n = 11), and mice that achieved normoglycemia after receiving islets intra-BM (dark gray; n = 47) or intraliver (gray; n = 32). Data are expressed as mean (line) and ±1 SD (area). aStatistical analysis was performed by tests of repeated measures ANOVA considering intervals of 2 months. bStatistical analysis was performed by tests of repeated measures ANOVA adjusted for islet number considering intervals of 2 months. *P < .05; **P > .001; ns = not significant. (B) Fasting glycemia (mg/dL), insulin (pM), glucagon (pM), β-cell function (HOMA2-B, %), and insulin resistance (HOMA-IR) in normal C57BL/6 mice (Ctrl, white), and mice that achieved normoglycemia after receiving islets intra-BM (dark gray; n = 47) or intraliver (gray; n = 32). Assays were performed 6 to 9 months after islet infusion. Data are expressed as box plots. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .05; **P < .001.
Graft survival and long-term glucose metabolism after islet transplantation in BM and liver. (A) Blood glucose profile of normal C57BL/6 mice (Ctrl, white; n = 11), and mice that achieved normoglycemia after receiving islets intra-BM (dark gray; n = 47) or intraliver (gray; n = 32). Data are expressed as mean (line) and ±1 SD (area). aStatistical analysis was performed by tests of repeated measures ANOVA considering intervals of 2 months. bStatistical analysis was performed by tests of repeated measures ANOVA adjusted for islet number considering intervals of 2 months. *P < .05; **P > .001; ns = not significant. (B) Fasting glycemia (mg/dL), insulin (pM), glucagon (pM), β-cell function (HOMA2-B, %), and insulin resistance (HOMA-IR) in normal C57BL/6 mice (Ctrl, white), and mice that achieved normoglycemia after receiving islets intra-BM (dark gray; n = 47) or intraliver (gray; n = 32). Assays were performed 6 to 9 months after islet infusion. Data are expressed as box plots. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .05; **P < .001.
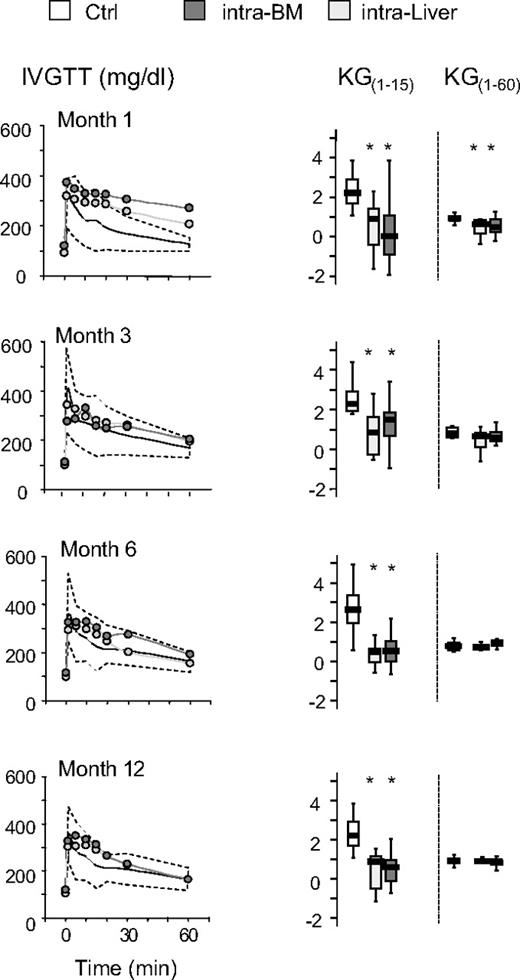
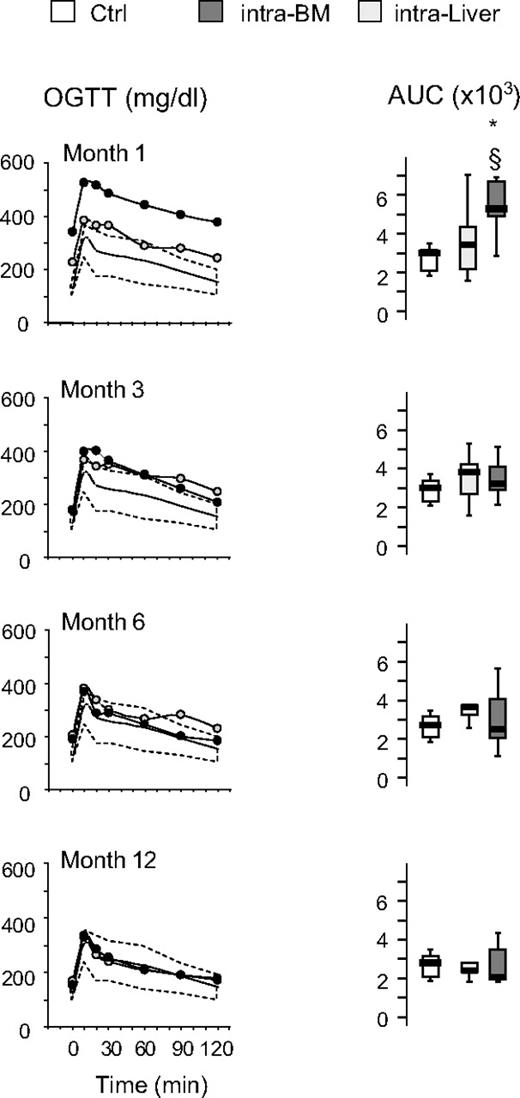
The time course of the effects of islet transplantation on glucose tolerance after IVGTT and OGTT is illustrated in Figures 4 and 5. No differences were observed in the euglycemic mice when liver versus bone marrow site was compared for both IVGTT and OGTT parameters. After 1 month, glucose elimination constant between 1 and 15 minutes (KG1-15) and 1 and 60 minutes (KG1-60) was significantly reduced in intra-BM and intraliver transplanted mice compared with control mice (Figure 4). Subsequently, in mice reaching euglycemia, the KG1-60 gradually improved in both intra-BM and intraliver models, reaching control values at 3 months after transplantation. The KG1-15 in both intra-BM and intraliver also improved, but remained significantly lower than in control mice during all the period of observation. OGTT at 1, 3, 6, and 12 months after transplantation showed that in mice that reached normoglycemia, the AUC for glucose eventually reached that of control mice for islet transplantation in both sites (Figure 5).
Blood levels of glucose and the glucose elimination constant (KG) during IVGTT after islet transplantation in BM and in liver. Mice were monitored by measuring glucose tolerance after IVGTT at 1, 3, 6, and 12 months after transplantation. Shown are IVGTT glycemic profile and glucose tolerance (KG1-15 and KG1-60) in mice that achieved normoglycemia (28 of 47 for intra-BM and 10 of 32 for intraliver). The yellow shaded area represents the range of values in healthy control mice (Ctrl, n = 11). Data are expressed as mean for glycemic profile and boxplots for glucose tolerance. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .01 versus control.
Blood levels of glucose and the glucose elimination constant (KG) during IVGTT after islet transplantation in BM and in liver. Mice were monitored by measuring glucose tolerance after IVGTT at 1, 3, 6, and 12 months after transplantation. Shown are IVGTT glycemic profile and glucose tolerance (KG1-15 and KG1-60) in mice that achieved normoglycemia (28 of 47 for intra-BM and 10 of 32 for intraliver). The yellow shaded area represents the range of values in healthy control mice (Ctrl, n = 11). Data are expressed as mean for glycemic profile and boxplots for glucose tolerance. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .01 versus control.
Blood levels of glucose and AUC for glucose during OGTT after islet transplantation in BM and liver. Glucose tolerance in an OGTT was measured at 1, 3, 6, and 12 months after islet transplantation into BM or liver. Shown are the OGTT glycemic profile and AUC for glucose in mice that achieved euglycemia (28 of 47 for intra-BM and 10 of 32 for intraliver). The yellow shaded area represents the range of values in healthy control mice (Ctrl, n = 11). Data are expressed as mean for glycemic profile and boxplots for glucose AUC. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .01 versus control.
Blood levels of glucose and AUC for glucose during OGTT after islet transplantation in BM and liver. Glucose tolerance in an OGTT was measured at 1, 3, 6, and 12 months after islet transplantation into BM or liver. Shown are the OGTT glycemic profile and AUC for glucose in mice that achieved euglycemia (28 of 47 for intra-BM and 10 of 32 for intraliver). The yellow shaded area represents the range of values in healthy control mice (Ctrl, n = 11). Data are expressed as mean for glycemic profile and boxplots for glucose AUC. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .01 versus control.
Morphologic study of islets 1 year after transplantation
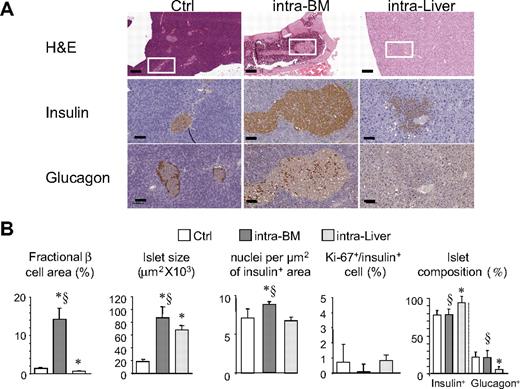
Islet morphology at 1 year after transplantation was evaluated in BM and liver in normoglycemic mice (6 mice per group) and in the pancreas of 6 control mice (Figure 6). Islets were detected in all femurs analyzed from the intra-BM group. Morphologically intact islets were present in femur diaphysis embedded in the cellular component of the BM (Figure 6A). For the intraliver model, several islets were present within the liver parenchyma and close to blood vessels of the portal tract. Generally, the islets in the liver did not maintain their original shape and appeared spread in the tissue (Figure 6A). As expected, the fractional β-cell area was significantly different between tissues, as follows: 14.2% in BM, 0.7% in liver, and 1.4% in control (P < .01 BM vs control and BM vs liver). Intra-BM transplants were characterized by approximately 4.5-fold increase in islet size compared with pancreatic islets (87 055 μm2 vs 18 596 μm2, P < .001, intra-BM vs control). An increased islet size in the liver (approximately 3.5-fold) was also observed (67 929 mm2, P < .05 vs control). Islets in the BM also had an increased density of β cells: BM, 8.9 ± 0.4 × 10−3 nuclei/μm2; liver, 6.7 ± 0.5 × 10−3 nuclei/μm2; control, 7.1 ± 1.2 × 10−3 nuclei/μm2 (P = .025, intra-BM vs control; P < .01, intra-BM vs liver transplantation). The frequency of β-cell replication (Ki67/insulin+ cells) at the 12-month time point was low in all groups (0.1 ± 0.6%, 0.7 ± 0.8%, and 0.8 ± 1.5%, respectively for intra-BM, intraliver, and control). A relative loss of glucagon-positive cells was observed in islets in the liver (5.8% vs 22% in islets within the pancreas, P < .01). This loss was not observed for islets in the bone marrow (21.5%). Of note, glucagon staining in BM islets appeared as single cells randomly scattered within the islets without any preferential distribution, whereas glucagon staining of islets in the pancreas showed the typical localization in the islet periphery.
Graft morphology. (A) Histologic appearance (top, ×5; scale bar, 400 μm; bottom, ×20; scale bar, 100 μm) of islets in control pancreas, BM, and liver 1 year after transplantation. (B) Fractional β-cell area, islet size, islet composition, β-cell proliferation, and nucleus density of insulin-positive area in mice reaching normoglycemia after islet transplantation in BM (n = 6) and liver (n = 6) 1 year after islet infusion, and in the pancreas of control mice (n = 6). Data are expressed as mean ± SD. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .01 versus control; §P < .05 versus intraliver.
Graft morphology. (A) Histologic appearance (top, ×5; scale bar, 400 μm; bottom, ×20; scale bar, 100 μm) of islets in control pancreas, BM, and liver 1 year after transplantation. (B) Fractional β-cell area, islet size, islet composition, β-cell proliferation, and nucleus density of insulin-positive area in mice reaching normoglycemia after islet transplantation in BM (n = 6) and liver (n = 6) 1 year after islet infusion, and in the pancreas of control mice (n = 6). Data are expressed as mean ± SD. Statistical analysis was performed using 1-way ANOVA test, followed by Bonferroni post hoc t test. *P < .01 versus control; §P < .05 versus intraliver.
Islet transplantation does not affect the hematopoietic activity of BM
To determine whether the presence of islets in the BM could affect hematopoietic activity, single-cell suspensions were prepared from BM by flushing the right femur (site of islet transplantation) and left femur (sham surgery) of 6 normoglycemic mice 1 year after islet transplantation. The cellularity of the right femur did not differ significantly from that of the left (7.9 ± 1.3 × 107 vs 7.8 ± 1.6 × 107 cells, P = .9). Analysis of BM cell subpopulations showed the same percentage of B220+, CD3+, Gr1+, Ter119+, c-Kit+, Sca1+, and Annexin V+ cells (supplemental Figure 4A). By CFC assay, the frequency of hematopoietic progenitors was 22.3 ± 7.3 × 10−4 BM cells and 25.5 ± 8.1 × 10−4 BM cells, respectively, for the right and left femur (P = .37). The types of hematopoietic colony-forming units (burst-forming unit–erythrocyte, colony-forming unit–granulocyte macrophage, colony-forming unit–granulocyte erythroid macrophage megakaryocyte) were equally represented (supplemental Figure 4A). We also evaluated peripheral blood cellularity 1 year after islet infusion in normoglycemic mice receiving islets in BM (n = 17), liver (n = 9), and in control mice (n = 11; supplemental Figure 4B). WBC and platelet counts were similar among the 3 groups. A moderate reduction in red blood cell counts, hemoglobin, and hematocrit was observed in mice receiving islets in the BM and in the liver, suggesting an association with the previous condition of diabetes rather than with the site of islet transplantation.
Next, we tested whether the presence of islets affects the capacity of BM to respond to virus-induced aplasia. At 1 year after transplantation, 6 of the normoglycemic mice that had received islet transplants in the BM were acutely infected with LCMV (BM-Tx-LCMV). LCMV is a noncytopathic mouse pathogen causing a systemic infection that targets most organs and tissues (including BM), but not pancreatic β cells.30 Acute LCMV infection is associated with an IFN-α/β–dependent BM aplasia that produces a transient pancytopenic state.28,31 The initial lymphopenia is rapidly reversed, such that within 1 week of LCMV exposure, the mice display lymphocytosis and clear the infection through a vigorous virus-specific cytotoxic T-lymphocyte (CTL) response.28,31 Results obtained in BM-Tx-LCMV were compared with those of age-matched, nontransplanted C57BL/6 mice that were either infected (LCMV) or not (control) (n = 6).
As shown in Figure 7A, all animals remained normoglycemic throughout the duration of the experiment (8 days). Weight loss was moderate and comparable between BM-Tx-LCMV and LCMV mice. Also comparable between the 2 groups of animals were the changes in platelet and WBC counts, which reached the expected nadir at day 4 postinfection and rebounded thereafter. In keeping with these results, there was no change in the hematopoietic activity of BM obtained from the femurs of BM-Tx-LCMV and LCMV mice, as can be inferred by the similar percentages of BM precursors detected at day 8 postinfection either by flow cytometry (Figure 7B) or CFC assay (data not shown). Note also that this was true when we compared the islet-containing right femur of BM-Tx-LCMV mice with the contralateral one in which islets were not present (Figure 7B). Importantly, BM-Tx-LCMV and LCMV mice cleared the virus with similar kinetics and by day 8 postinfection mounted a LCMV-specific CTL response that was quantitatively identical (Figure 7C). Altogether, the results aforementioned demonstrate that islets transplanted in BM do not affect normal or virus-induced hematopoietic activity.
Hematopoietic activity in intra-BM islet-infused mice after infection with LCMV. (A) Mice receiving islets intra-BM were infected at approximately 1 year of age with 2 × 105 plaque-forming units of LCMV. Glycemia, platelet, and WBC counts were measured either daily or every other day. (B) Femurs collected from intra-BM islet-transplanted LCMV-treated mice and from controls (age-matched C57BL/6 mice either infected with LCMV or not) were harvested on day 8 postinfection and monitored for the relative content of BM precursors and the presence of insulin-producing β cells. (C) Viral titers in blood harvested at the indicated time points after infection were measured by a reverse transcription–polymerase chain reaction–based assay with a detection limit of 103 copies of LCMV RNA (genome equivalents [GE]) per mL of total blood. Results are expressed as means of triplicate measurements ± SD. Assessment by intracellular IFN-γ staining of the percentage of glycoprotein 33-specific CD8+ T cells recovered from spleens harvested at day 8 postinfection. Results are expressed as mean ± SD.
Hematopoietic activity in intra-BM islet-infused mice after infection with LCMV. (A) Mice receiving islets intra-BM were infected at approximately 1 year of age with 2 × 105 plaque-forming units of LCMV. Glycemia, platelet, and WBC counts were measured either daily or every other day. (B) Femurs collected from intra-BM islet-transplanted LCMV-treated mice and from controls (age-matched C57BL/6 mice either infected with LCMV or not) were harvested on day 8 postinfection and monitored for the relative content of BM precursors and the presence of insulin-producing β cells. (C) Viral titers in blood harvested at the indicated time points after infection were measured by a reverse transcription–polymerase chain reaction–based assay with a detection limit of 103 copies of LCMV RNA (genome equivalents [GE]) per mL of total blood. Results are expressed as means of triplicate measurements ± SD. Assessment by intracellular IFN-γ staining of the percentage of glycoprotein 33-specific CD8+ T cells recovered from spleens harvested at day 8 postinfection. Results are expressed as mean ± SD.
pQCT measurements: cross-sectional in vivo evaluation of graft-bearing and sham-treated contralateral femurs
Right femur (site of islet transplantation) and left femur (sham surgery) of 11 normoglycemic mice were analyzed 9 months after islet transplantation. pQCT analysis showed a significant decrease (P < .01) of the cortical density that was localized at all cross-sectional scanning sites (supplemental Figure 5). Cortical thickness was found slightly, but significantly decreased only at the most distal section, that is, the closest section to graft implant site. None of the other density and size parameters both at the expenses of the cortical and trabecular compartments were significantly affected by the presence of the graft (see supplemental Table 1). A nonsignificant trend toward decrement of the SSI was observed in the right femur.
Discussion
The liver is the current site for pancreatic islet transplantation, but presents important limitations. The potential solution to this problem is to establish an alternative site for clinical islet transplantation. We asked whether pancreatic islets could be engrafted in the BM, an easily accessible and widely distributed transplant site that may lack the limitations seen in the liver. The results show that pancreatic islets can be efficiently engrafted into BM. This is the first report of BM as a successful site for seeding pancreatic islets in diabetic recipients. A single work previously described injection of islet in BM of nondiabetic rats with the detection of positive staining for insulin up to 21 days after infusion without any functional evaluation.32
In our study, both the percentage and the timing in reversal of hyperglycemia were superior after BM infusion compared with intrahepatic infusion using the minimal mass model. Moreover, with the exception of a small delay in gaining normal glucose tolerance after OGTT, the quality of glucose metabolism in mice that reached normoglycemia via intra-BM islet infusion was similar to that achieved by islet transplant into the liver for all the parameters evaluated (fasting and not fasting glycemia, blood insulin, HOMA-B, and glucose tolerance after IVGTT). Based on our results, we can conclude that the BM site for islet transplantation has a higher probability to reach euglycemia (2.5-fold increase in a multivariate analysis) than the liver without compromising the quality of glucose metabolism. This is relevant because the process of intrahepatic infusion was traditionally considered optimal due to the supposition that insulin is delivered more physiologically after intraportal transplantation.32-35 However, this argument has recently been challenged by experimental studies showing that intraportally transplanted islets respond to glucose stimulation only when perfused via the hepatic artery; no response is observed after challenge via the portal vein.36 There are also reports on alterations in islet function after intraportal islet transplantation, such as a defective glucagon response to hypoglycemia37-39 and defective glucose-stimulated insulin release.40
In relation to the site of engraftment, islet morphology and cellular composition showed profound changes after transplantation. Islets transplanted in BM showed an increased size with a compact morphology. The most likely explanation for the increased size lies in the method of cell isolation and infusion. Before the introduction into BM, islets are packed, and this could allow the formation of aggregates. This was also supported by the fact that the increase in islet size was not associated with increased cell volume. Nevertheless, without time course studies, we cannot exclude an increased replication of cells within the transplanted islets leading to the increased size observed at 12 months after transplantation. The fraction of α and β cells in islets engrafted in BM was similar to control islets. In contrast, the fraction of α cells was markedly decreased in the intraportally transplanted islets. The reason for this selective decrease of the α-cell fraction is unknown, but is consistent with the observed decreased glucagon response to hypoglycemia seen in humans after islet transplantation.37
The process of intrahepatic infusion is currently considered safe, although there is a low risk of portal vein thrombosis and elevated portal pressures, in addition to bleeding from the percutaneous hepatic puncture site.41-44 From a clinical viewpoint, BM seeding has the potential of being a less invasive, ambulatory procedure with almost unlimited opportunities for repeated implantation, as well as a low-risk, easy-access site for graft biopsies. This potential was recently confirmed by the clinical experience of direct intrabone cord-blood transplantation. Frassoni et al recently reported a cohort of 32 patients receiving infusion of 20 mL cell suspension in superior-posterior iliac crest.45 The infusion was performed easily without resistance. Moreover, no side effects, such as pain, hemorrhage, or infections, were recorded, suggesting that the same technique could be used for islet infusion.
Because it was suggested that hyperinsulinemia might contribute to cancer development through the growth-promoting effect of elevated levels of insulin, it is possible that intra-BM islet transplantation could increase the risk of proliferative disease. For this reason, we evaluated the impact of islets on hematopoietic activity of BM. One year after islet infusion, the cellularity, the histologic appearance, the analysis of cell subpopulation, and the progenitor cell frequency were unaffected by the presence of islets in the BM. It should also be underlined that a potential tumorogenic risk from local hyperinsulinemia is also present after intrahepatic islet infusion. Indeed, in diabetic bio breeding rats receiving intraportal islet transplants with long-term partial function, there was a remarkably high incidence of adenomas, and even hepatocellular carcinoma.46 The initial stages in the process leading to these pathologic conditions were hepatocyte steatosis and glycogen deposits in the vicinity of the transplanted islets, findings that are analogous to those reported in patients from several transplant centers.47,48
We also took into consideration the consequences of BM islet infusion on the capacity to respond to virus-induced aplasia and the bone structure. Islets in BM of LCMV-infected mice did not affect hematopoietic activity consequent to aplasia nor CTL-mediated viral clearance. These results also suggest that islets in BM are capable of sustaining those metabolic changes that are likely to occur during the rapid expansion of a very robust adaptive immune response (ie, by day 8 postinfection, secondary lymphoid organs of LCMV-infected mice are much larger in size, and approximately 50% to 70% of all CD8+ T cells are LCMV specific49 ). The islet graft did cause minor alterations of the bone microenvironment with a subsequent volumetric bone density loss at the cortical compartment facing the grafted pancreatic islets. Because bone development predicts bone mass accrual as a consequence of a modeling process during growth, it is conceivable that the decreased cortical density is the outcome of enhanced osteoclastogenesis rather than a reduced osteoblastogenesis, as the latter should have resulted in a reduced outward shift of the bone cortex. Regardless of the mechanism, the issue is whether bone resorption in the cortical compartment is a potential contraindication to the use of BM as a site of engraftment. From a quantitative point of view, the bone loss was between 4 and 6% compared with the controlateral femur after 9 months. Moreover, it should be considered that in an eventual clinical application, the site of infusion would be the iliac crest. Because iliac crest is not a weight-bearing skeletal site, a limited loss of its cortical density is not expected to have important clinical consequences. Moreover, bone per se might constitute the critical microenvironment for pancreatic islet function and survival. It has been in fact shown that picomolar amounts of osteocalcin, the osteoblast-specific secreted molecule, are sufficient to enhance the expression of the insulin genes and β-cell proliferation markers in cell-based assays using isolated pancreatic islets.50 For all safety considerations and indeed for evaluation of the suitability of the BM site in humans, it should be considered that islet transplantation is usually performed with allogenic islets and under immunosuppression. Islet function after transplantation is affected by immunosuppression, and our preliminary results suggest that this is also the case when syngeneic islets are transplanted in the BM (L.P., unpublished observations).
In conclusion, we show that pancreatic islets can be engrafted into the BM, thus opening a research line with potentially significant clinical impact not only for the treatment of diabetes, but for other diseases amenable to treatment with cellular transplantation. Because the BM as a site for pancreatic islet grafts can be clinically applicable and, in theory, can solve many of the problems encountered with the intrahepatic location, further research is warranted by the initial findings presented in this study to determine whether the results can be reproduced in large animals and eventually in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Claudio Doglioni (Department of Pathology, San Raffaele Scientific Institute) and Dr Fabio Ciceri (Unit of Hematology and Bone Marrow Transplantation, Department of Oncology, San Raffaele Scientific Institute) for the useful discussion of these experimental results.
This work was supported by the European Union (DIAPREPP Project, HEALTH-F2-2008-202013), Telethon Italy and the Juvenile Diabetes Research Foundation (JT01Y01), Italian Telethon Foundation (Telethon Institute for Gene Therapy grant) and CARIPLO Foundation, and the Deutsche Forschungsgemeinschaft-Center for Regenerative Therapies Dresden, Cluster of Excellence at Dresden University of Technology. V.S. is enrolled as a PhD student at the Ludwig-Maximilians University of Munich.
Authorship
Contribution: E.C. performed research; R.M. performed research; A.M. performed research; V.S. analyzed data; G.F. designed research; C.W.L. performed research; E.M. performed research; A.R. designed research; M.P. analyzed data; G.S. performed research; L.G.G. designed research and wrote the paper; E.B. designed research and wrote the paper; and L.P. designed research, performed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lorenzo Piemonti, Diabetes Research Institute, San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milano, Italy; e-mail: piemonti.lorenzo@hsr.it.
References
Author notes
E.C. and R.M. contributed equally to this work.

![Figure 7. Hematopoietic activity in intra-BM islet-infused mice after infection with LCMV. (A) Mice receiving islets intra-BM were infected at approximately 1 year of age with 2 × 105 plaque-forming units of LCMV. Glycemia, platelet, and WBC counts were measured either daily or every other day. (B) Femurs collected from intra-BM islet-transplanted LCMV-treated mice and from controls (age-matched C57BL/6 mice either infected with LCMV or not) were harvested on day 8 postinfection and monitored for the relative content of BM precursors and the presence of insulin-producing β cells. (C) Viral titers in blood harvested at the indicated time points after infection were measured by a reverse transcription–polymerase chain reaction–based assay with a detection limit of 103 copies of LCMV RNA (genome equivalents [GE]) per mL of total blood. Results are expressed as means of triplicate measurements ± SD. Assessment by intracellular IFN-γ staining of the percentage of glycoprotein 33-specific CD8+ T cells recovered from spleens harvested at day 8 postinfection. Results are expressed as mean ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/20/10.1182_blood-2009-03-209973/4/m_zh89990944750007.jpeg?Expires=1771332335&Signature=f3SZLMhnYM1oa1gtTqeBXI4CYUZGDvbyQ2AC1b5ZLVz9Va7~UQh10SRa2ZCt7eR~Fm78fOTRKaJojMCWdTfjUvDBwtxOvqwr7dOni5ktXTVmKl2TCRNruANeo~ZR8quyxA-n8kgU4Jq~9SMiCeo4IkIMsUquj98~sxMLtEbZrZegBiK2X78PJYjngyak~vdofZHyiNRZ69HI04zRpv9nBdOOoeZxpb5Cz1QzVnU-jAh9d7iVvEfBPMIeRPy3cR5-yY6ZzXCPUb3898ZCVsuaMcDLdk8sHijyyO~UPM7USwRNBvWsuOpFPMkk2K6BT2~pYZYFFYVTc6v8JNJ5OYv77w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)