Abstract
Production of recombinant B-domain–deleted canine factor VIII (cFVIII-BDD) unexpectedly revealed superior protein yields with 3-fold increased specific activity relative to human FVIII-BDD (hFVIII-BDD). We also determined that activated cFVIII-BDD is more stable than activated hFVIII-BDD. Furthermore, cFVIII-BDD is efficient at inducing hemostasis in human plasma containing FVIII inhibitors. Infusion of cFVIII-BDD in hemophilia A dogs resulted in correction of the disease phenotype with a pharmacokinetic profile similar to clinical experience with hFVIII-BDD. Notably, immune tolerance challenges with cFVIII-BDD in young and adult hemophilia A dogs did not induce the formation of neutralizing or nonneutralizing antibodies to cFVIII. These data establish the framework to quantitatively investigate the efficacy and safety in preclinical studies of novel therapies for hemophilia A.
Introduction
Hemophilia A (HA) is an X-linked bleeding disease resulting from a functional factor VIII (FVIII) deficiency affecting 1 in 5000 males worldwide. For several decades, the HA dog model has been the most extensively used for preclinical studies.1 Notably, in 2 strains of dogs, the underlying mutation consists of an inversion in intron 22 of the FVIII gene that is analogous to the most common human mutation.2 This model faithfully replicates the human disease at both genotypic and phenotypic levels.3,4 To date, there is no characterization of the cFVIII protein resulting from difficulties in purifying large amounts from canine plasma and to the relative poor performance in recombinant FVIII expression systems in general. Although the cFVIII cDNA sequence has a high sequence identity to human FVIII (hFVIII),5 adult HA dogs develop immune responses on exposure to hFVIII that preclude the assessment of the efficacy and safety of potential novel therapies for HA. Notably, among humans, even small nucleotide changes in the hFVIII gene may predispose to inhibitor formation.6 To overcome these limitations, we established a heterologous expression system for cFVIII. Our findings uncovered unforeseen enhanced biologic properties of the protein. This work fills an important void for the study of cFVIII biologic properties and immune responses in HA dogs.
Methods
Production and characterization of recombinant cFVIII-BDD
Permission was obtained from the Institutional Animal Care and Use Committee of the University of Pennsylvania and the Children's Hospital of Philadelphia for all studies. cFVIII-BDD7 and hFVIII-BDD8 were expressed in baby hamster kidney cells and purified as previously described (supplemental data and supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).8-11 Canine and human FVIII-BDD protein concentrations were determined by absorbance at 280 nm using an extinction coefficient (E280, 1%) of 1.60 and molecular weight of 160 000; these values, obtained with porcine FVIIIa, were assumed to be the same as canine and human FVIII-BDD.12 Protein specific activity was determined by activated partial thromboplastin time (aPTT) with minor modifications.13 Decay of activated FVIII activity was monitored by purified component assay using reconstituted human factor Xase complex and plasma models as previously described.11 N-terminal sequencing was determined in the laboratory of Dr Alexander Kurosky and Steven Smith at University of Texas Medical Branch (Galveston, TX). Enzymatic cleavage of N-linked glycans was carried out using recombinant N-glycosidase F (Boehringer Mannheim) as reported before.14
cFVIII antigen, activity, and antibody assays
Purified cFVIII-BDD was used as a standard for the quantitation of the activity by Chromogenix Coatest SP4 FVIII (Diapharma). cFVIII-BDD protein was used for the generation of a series of rabbit anti-cFVIII-BDD polyclonal and murine monoclonal anti-cFVIII-BDD antibodies (Green Mountain Antibodies; supplemental Figure 1). Anti-cFVIII antibodies were detected by Bethesda assay15 or by cFVIII-specific IgG antibodies by enzyme-linked immunosorbent assay (supplemental data). Half-life and recovery were calculated as previously described.16,17
Results and discussion
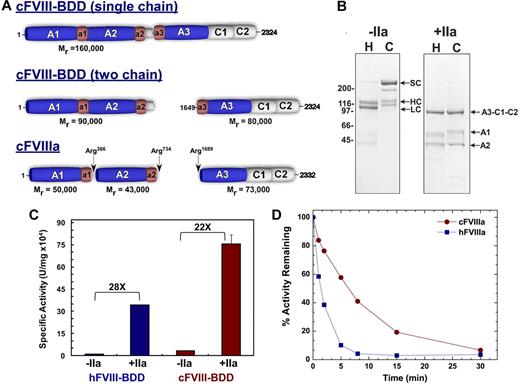
Using identical expression systems, we found that cFVIII-BDD typically yields 0.5 mg/L, which is 3-fold higher than hFVIII-BDD (0.16 mg/L). Notably, purified cFVIII-BDD existed predominantly as a single-chain protein (> 75% of total), whereas, as expected, hFVIII-BDD was primarily a heterodimer (Figure 1A-B). The amino acid recognition sequence for PACE/furin in cFVIII (HHQR)5 differs from human and porcine FVIII (RHQR) and may explain the predominant single-chain form of cFVIII.18 After treatment with thrombin, cFVIII-BDD was properly activated to yield the heterotrimer. Minor differences in the migration pattern of the A1 domain were noted between hFVIII-BDD and cFVIII-BDD (Figure 1B). However, removal of N-linked glycan resulted in similar migration of the A1 domains (data not shown). These data indicate that either the glycosylation structure on the A1 domains is different or that possibly only one site in the human A1 domain is glycosylated. Moreover, N-terminal sequencing of all relevant bands yielded the expected results (data not shown). Using a 1-stage aPTT, the specific activity of cFVIII-BDD (33 926 ± 675 U/mg) was approximately 3-fold higher than hFVIII-BDD (12 345 ± 787 U/mg; P < .001). Similar findings were obtained after thrombin activation of canine and human FVIII in the 2-stage aPTT (756 754 ± 60 592 vs 343 066 ± 2090 U/mg, P < .003) yielding an activation quotient of 22 and 28 for canine and human, respectively (Figure 1C). Typically, a low activation quotient represents contamination with activated forms of the protein and results in false high protein activity. These findings were consistent using 3 separate cFVIII-BDD preparations. Taken together, these data using purified FVIII protein support the conclusions that cFVIII has an elevated intrinsic specific activity.
Biochemical characterization of FVIII-BDD. (A) Canine (c) FVIII-BDD is predominantly synthesized as 160 000 single chain protein with a smaller proportion being processed as a heterodimer. Thrombin (IIa) cleaves cFVIII-BDD at the indicated sites to yield activated cFVIII. (B) Protein purity was assessed by loading 4 μg of human FVIII-BDD (H) and cFVIII-BDD (C) on a reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by staining with Coomassie blue (left; −IIa). FVIII-BDD (H or C; 800nM) was incubated with IIa (+IIa; 5nM) for 10 minutes, and the resulting activated FVIII was run on a reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (right, +IIa). The various domains of FVIII are indicated. SC indicates single chain; HC, heavy chain; LC, light chain; A3-C1-C2 (73 kDa), A1 (50 kDa), and A2 (43 kDa). (C) The specific activity of cFVIII-BDD and hFVIII-BDD was compared using a 1- or 2-stage aPTT in human-deficient plasma. For the 2-stage assay (+IIa), FVIII-BDD (human or canine; 20nM) in 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/150mM NaCl/5mM CaCl2/0.01% Tween 80, pH 7.4 (assay buffer) was intentionally activated with IIa (40nM) for 30 seconds at 25°C. Activated FVIII was immediately diluted into assay buffer with 0.1% albumin and then subsequently added to the aPTT clotting assay. In either the 1- (−IIa) or 2-stage aPTT (+IIa), the specific activity of cFVIII-BDD was 3-fold higher than hFVIII-BDD. The activation quotient was 22 for cFVIII and 28 for hFVIII. (D) A purified Xase assay was used to assess A2-domain stability. The Xase assay was performed by activating 20nM cFVIII-BDD or hFVIII-BDD with 40nM IIa for 30 seconds at 25°C. The reaction was stopped by adding 60nM hirudin. At various time points after activation, FVIIIa (0.2nM, final) was added to the Xase complex (hFIXa, 2nM; hFX, 300nM; and phospholipids, 20μM; phosphatidylcholine/phosphatidylserine, 75:25), and activation was measured by monitoring FXa generation using a chromogenic substrate.
Biochemical characterization of FVIII-BDD. (A) Canine (c) FVIII-BDD is predominantly synthesized as 160 000 single chain protein with a smaller proportion being processed as a heterodimer. Thrombin (IIa) cleaves cFVIII-BDD at the indicated sites to yield activated cFVIII. (B) Protein purity was assessed by loading 4 μg of human FVIII-BDD (H) and cFVIII-BDD (C) on a reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by staining with Coomassie blue (left; −IIa). FVIII-BDD (H or C; 800nM) was incubated with IIa (+IIa; 5nM) for 10 minutes, and the resulting activated FVIII was run on a reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (right, +IIa). The various domains of FVIII are indicated. SC indicates single chain; HC, heavy chain; LC, light chain; A3-C1-C2 (73 kDa), A1 (50 kDa), and A2 (43 kDa). (C) The specific activity of cFVIII-BDD and hFVIII-BDD was compared using a 1- or 2-stage aPTT in human-deficient plasma. For the 2-stage assay (+IIa), FVIII-BDD (human or canine; 20nM) in 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/150mM NaCl/5mM CaCl2/0.01% Tween 80, pH 7.4 (assay buffer) was intentionally activated with IIa (40nM) for 30 seconds at 25°C. Activated FVIII was immediately diluted into assay buffer with 0.1% albumin and then subsequently added to the aPTT clotting assay. In either the 1- (−IIa) or 2-stage aPTT (+IIa), the specific activity of cFVIII-BDD was 3-fold higher than hFVIII-BDD. The activation quotient was 22 for cFVIII and 28 for hFVIII. (D) A purified Xase assay was used to assess A2-domain stability. The Xase assay was performed by activating 20nM cFVIII-BDD or hFVIII-BDD with 40nM IIa for 30 seconds at 25°C. The reaction was stopped by adding 60nM hirudin. At various time points after activation, FVIIIa (0.2nM, final) was added to the Xase complex (hFIXa, 2nM; hFX, 300nM; and phospholipids, 20μM; phosphatidylcholine/phosphatidylserine, 75:25), and activation was measured by monitoring FXa generation using a chromogenic substrate.
After activation, FVIIIa rapidly loses activity because of A2-domain dissociation from the A1/A2/A3-C1-C2 heterotrimer. Purified cFVIII-BDD or hFVIII-BDD was rapidly activated (∼ 30 seconds) with thrombin, and residual cofactor activity was monitored over time. Using either a purified component assay (Figure 1) or clotting assay (data not shown), we found that the half-life of cFVIIIa was 3-fold longer than hFVIIIa. These findings suggest that cFVIIIa exhibits increased affinity for the A2-domain compared with hFVIIIa. Although these data could, in part, account for the high specific activity of cFVIIIa, both porcine18 and murine proteins11 also have enhanced A2-domain stability compared with hFVIIIa but apparently have equivalent specific activity to hFVIII. Thus, it is possible that the increased specific activity of cFVIII is the result of the single chain protein or other factors rather than A2-domain stability.
To test the efficacy and safety of the cFVIII-BDD, we injected a series of adult and neonate HA dogs. In these dogs, no circulating FVIII antigen was detected, which is consistent with humans with the analogous FVIII mutation. In normal dogs, cFVIII levels are80 to 130 ng/mL, which is comparable with human levels (100-200 ng/mL) and with cFVIII levels previously described.19
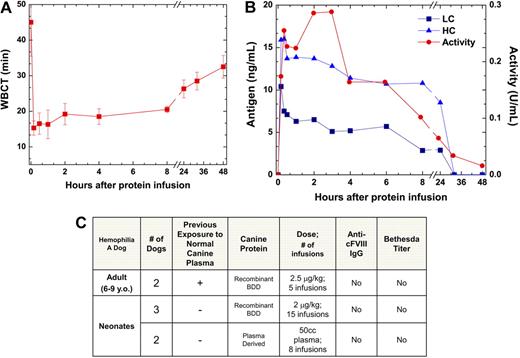
HA dogs received cFVIII-BDD at doses of 2.5 μg/kg every 30 days for 2 to 4 months, and serial plasma samples were collected through 4 weeks after each protein injection. cFVIII-BDD was functional as evidenced by the shortening of the whole blood clotting time (WBCT) and increased cFVIII clotting activity (Figure 2). The recovery of the protein, measured at 5 to 10 minutes (n = 5 infusions) after injection, was excellent, reaching levels of 71.8% plus or minus 9.2%. There was a good correlation between cFVIII activity and antigen levels (Figure 2B). The levels of cFVIII slowly declined after the infusion and returned to baseline within 48 to 56 hours with a calculated half-life of 12 to 14 hours. There was no local or systemic toxicity and no evidence of pathologic activation of coagulation. To date, an additional 16 HA dogs received either a single or multiple injection at doses ranging from 2.0 μg/kg to 200 μg/kg for a combined total of approximately 6 mg of cFVIII-BDD with successful control of hemostasis. Together these data demonstrate that cFVIII-BDD is safe and efficacious in inducing sustained hemostasis in vivo and has a protein half-life comparable with the pharmacokinetics of hFVIII-BDD in HA dogs and from clinical experience in humans.20
Canine FVIII-BDD is functional and does not induce an immune response in HA dogs. (A) WBCT after 3 injections of cFVIII-BDD in an HA dog (mean ± SD). The WBCT shortened within 5 minutes of the protein infusion from more than 45 minutes (baseline) to 13 to 16.5 minutes (normal range, 8-12 minutes). (B) FVIII antigen and clotting activity after intravenous injection of cFVIII-BDD. For one protein infusion of the same dog, cFVIII activity was determined by Coatest assay and antigen levels were determined by enzyme-linked immunosorbent assay specific for the cFVIII heavy (HC) or light chain (LC). The Coatest was performed using purified cFVIII as a standard. One unit is defined as 100 ng/mL. (C) Monitoring antibody and inhibitor formation to cFVIII-BDD in HA dogs. In addition to the adult dogs, neonatal naive animals that had not previously been exposed to normal canine plasma were treated with cFVIII-BDD. IgG represents both IgG1 and IgG2 data.
Canine FVIII-BDD is functional and does not induce an immune response in HA dogs. (A) WBCT after 3 injections of cFVIII-BDD in an HA dog (mean ± SD). The WBCT shortened within 5 minutes of the protein infusion from more than 45 minutes (baseline) to 13 to 16.5 minutes (normal range, 8-12 minutes). (B) FVIII antigen and clotting activity after intravenous injection of cFVIII-BDD. For one protein infusion of the same dog, cFVIII activity was determined by Coatest assay and antigen levels were determined by enzyme-linked immunosorbent assay specific for the cFVIII heavy (HC) or light chain (LC). The Coatest was performed using purified cFVIII as a standard. One unit is defined as 100 ng/mL. (C) Monitoring antibody and inhibitor formation to cFVIII-BDD in HA dogs. In addition to the adult dogs, neonatal naive animals that had not previously been exposed to normal canine plasma were treated with cFVIII-BDD. IgG represents both IgG1 and IgG2 data.
The use of these outbred immunocompetent HA dogs provides an ideal model to test the immunogenicity of cFVIII-BDD protein in both naive neonates and adult dogs previously exposed to plasma-derived cFVIII. These dogs do not develop antibodies to cFVIII on infusion of plasma-derived cFVIII. Here, in adult dogs, no antibodies to cFVIII-BDD were detected by Bethesda assay or cFVIII-specific IgGs after repetitive exposure to the protein (Figure 2C). Furthermore, neonate dogs (n = 3) exclusively exposed to cFVIII-BDD or small amounts of plasma-derived FVIII (n = 2) also did not develop antibodies to cFVIII. In an HA dog with an inhibitor to plasma-derived cFVIII, inhibitor titers of 4 Bethesda units corresponds to 3000 to 4000 ng/mL IgG2. These data are in contrast to the strong immune responses of adult HA dogs to hFVIII characterized by long-lasting antibody to hFVIII after exposure to the protein or after delivery of hFVIII gene- or cell-based therapies.1,17,20,21 Thus, cFVIII-BDD presents no immunogenicity in this pivotal HA dog model, which is essential for determining long-term efficacy and safety of novel therapeutic strategies for HA.
We sought to compare the rates of inactivation of cFVIII-BDD with hFVIII-BDD in human plasma containing inhibitors to FVIII. The recovery of cFVIII after incubation with inhibitors was 40% to 45% higher than hFVIII (supplemental Figure 2). The higher survival of cFVIII in the presence of human inhibitors further supports the investigation of cFVIII as a potential bypass strategy for hemophilia.
The recombinant expression of cFVIII-BDD allowed us to generate large amounts of protein (> 20 mg; 4 preparations), develop valuable antibodies, and begin to unravel intrinsic properties of the protein that may impact the development of hemophilia treatment. The underlying mechanisms for the enhanced biologic activity of cFVIIIa are not entirely clear. However, it could partially result from the secretion of cFVIII as a single-chain protein; hFVIII variants with a canine PACE/furin cleavage site may help define whether these modifications would improve production and stability of the recombinant protein. Furthermore, a detailed analysis of Xase complex assembly in kinetic characterization with canine FVIIIa could shed light on its apparent increased specific activity compared with hFVIIIa.
The efficacy and safety data from studies on noninhibitor prone HA dogs demonstrate that cFVIII-BDD is an attractive option for the treatment of bleeds and for prophylaxis in dogs during complex or invasive procedures. The ability to detect nonneutralizing IgG antibodies in addition to neutralizing antibodies provides the opportunity to elucidate conflicting findings in gene- or cell-based therapy in these dogs.22-25 The more comprehensive phenotypic characterization of the HA dogs is now feasible and further improves the relevance of preclinical studies for a new generation of gene- and cell-based therapies for hemophilia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Hemophilia Association of New York (V.R.A.) and the National Institutes of Health (HL084220, V.R.A.; HL083017, H.H.K.; HL88010 and HL74124, R.M.C.; and R2414L063098, T.C.N.).
National Institutes of Health
Authorship
Contribution: V.R.A. and R.M.C. designed the study; D.E.S., C.F.F., and R.T. performed the research and analyzed data; H.H.K. provided the canine FVIII cDNA; A.S. and C.F.F. purified the recombinant protein; T.C.N. and E.P.M. performed the HA dog studies; and R.M.C., D.E.S., and V.R.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valder R. Arruda, University of Pennsylvania School of Medicine, Children's Hospital of Philadelphia, 302F Abramson Research Center, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: arruda@email.chop.edu.
References
Author notes
D.E.S. and C.F.F. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal