Abstract
Deferoxamine (DFO) is a high-affinity Fe (III) chelator produced by Streptomyces pilosus. DFO is used clinically to remove iron from patients with iron overload disorders. Orally administered DFO cannot be absorbed, and therefore it must be injected. Here we show that DFO induces ferritin degradation in lysosomes through induction of autophagy. DFO-treated cells show cytosolic accumulation of LC3B, a critical protein involved in autophagosomal-lysosomal degradation. Treatment of cells with the oral iron chelators deferriprone and desferasirox did not show accumulation of LC3B, and degradation of ferritin occurred through the proteasome. Incubation of DFO-treated cells with 3-methyladenine, an autophagy inhibitor, resulted in degradation of ferritin by the proteasome. These results indicate that ferritin degradation occurs by 2 routes: a DFO-induced entry of ferritin into lysosomes and a cytosolic route in which iron is extracted from ferritin before degradation by the proteasome.
Introduction
Ferritin, a multimeric protein composed of 24 monomers, is capable of storing up to 4500 atoms of iron.1 Storage of iron within ferritin serves 2 important functions. First, iron stored within ferritin is in a redox inactive form, which reduces iron toxicity resulting from generation of oxygen radicals.2 Second, ferritin iron can be mobilized and used for metabolic purposes under conditions of cellular iron demand. Synthesis of ferritin is regulated posttranslationally to rapidly provide iron storage capacity when cytosolic iron is high.3 In conditions that lead to iron demand, iron stored in ferritin can be recovered and ferritin chains degraded. Studies using expression of the iron exporter ferroportin to deplete cytosolic iron indicated that iron was released from ferritin shells in the cytosol and apoferritin degraded by the proteasome.4 Alternatively, studies using the iron chelator deferoxamine (DFO) demonstrated that ferritin was degraded in lysosomes and that iron release was dependent on ferritin degradation.5,6 The mechanisms determining whether ferritin degradation occurs in the lysosomes or by the proteasome have not been defined. To address this issue, we examined the effects of 2 membrane-permeable iron chelators as well as DFO on ferritin degradation. Our results indicate that cell-permeable iron chelators induce ferritin degradation via the proteasome. DFO, which is poorly permeable, leads to ferritin degradation by lysosomes through induction of autophagy. The induction of autophagy is due to the presence of DFO within the lysosomal lumen. A combination of iron chelators is thought to be more effective for iron chelation in specific organs.7 The different effects of DFO and the permeable iron chelators on ferritin degradation might contribute to the efficacy of combination chelator therapy in iron overload disease.
Methods
Cells and media
HEK293T-Fpn cells, a stable line expressing Fpn-GFP under the regulation of the ecdysone promoter, were grown and induced as described previously.8 HeLa cells were grown in Dulbecco modified Eagle medium with 10% fetal bovine serum and transfected with pDynaminK44A-EGFP or pCMV-Fpn-FLAG using Nucleofector technology (Amaxa), according to the manufacturer's directions. Wild-type (Wt) and Lamp1−/−,2−/− fibroblast were a generous gift from Dr Paul Saftig (University of Kiel) and were grown in Dulbecco modified Eagle medium with 10% fetal bovine serum.
Ferritin measurement
Cells were incubated with ferric ammonium citrate (FAC; 10μM Fe) for 24 hours in the absence or presence of DFO (100μM; Ciba-Geigy), deferriprone and desferasirox or chloroquine (100μM; Sigma-Aldrich), or MG132 (10μM; Sigma-Aldrich). Deferriprone and desferasirox were a generous gift from Dr Prem Ponka (McGill University). Rapamycin (Sigma-Aldrich) was used at the final concentration of 100nM. Cellular proteins were extracted with 150mM NaCl, 10mM EDTA, 10mM Tris (pH 7.4), 1% Triton X-100, and protease inhibitor cocktail (Roche). Ferritin levels were measured as described.8 Protein concentration was determined by bicinchoninic acid assay (Pierce).
Immunofluorescence
Cells were fixed with 3.7% formaldehyde, permeabilized in PBS containing 1% bovine serum albumin, 0.1% saponin, and incubated with rabbit anti-LC3B (1:200; Cell Signaling) for 60 minutes at room temperature, followed by Alexa 488–conjugated goat anti–rabbit antibody (1:750; Invitrogen) and nuclei were stained with 4′,6-diamidino-2-phenylindole (blue; Invitrogen). We performed 3 independent experiments examining more than 5 fields/sample with 10 to 15 cells/field. Cells were visualized using an epifluorescence microscope (Olympus) with a 60× oil-immersion objective. Images were acquired using picture framer analysis software (Olympus).
Percoll gradients and β-N-acetylhexoseaminidase analysis
Localization of ferritin within lysosomes was analyzed by subcellular fractionation as described previously.4 Briefly, cells were homogenized in buffer (250mM sucrose, 20mM HEPES, 0.5mM EGTA, pH 7.2, KOH) using a ball-bearing homogenizer. The homogenate was centrifuged at 800g for 5 minutes at 4°C to obtain a postnuclear supernatant that was applied to 30% Percoll, which was centrifuged at 59 000g for 30 minutes. Gradients were fractionated and ferritin was measured by enzyme-linked immunosorbent assay (ELISA), and β-N-acetylhexoseaminidase was assayed colorimetrically as described previously.4
Results
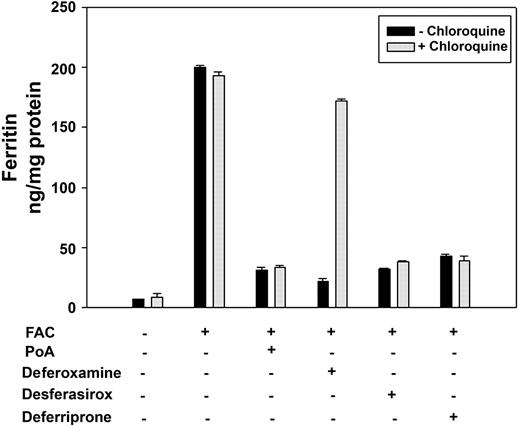
Evidence that ferritin may be degraded in the lysosome is based on the observation that inhibiting lysosome proteolysis, either by inhibiting protease activity with leupeptin or by affecting lysosomal pH with chloroquine, prevents ferritin loss in DFO-treated cells.6 We confirmed that DFO-induced ferritin loss is prevented by chloroquine treatment (Figure 1). Ferritin degradation induced by expression of the iron exporter ferroportin (Fpn) or by addition of the oral iron chelators desferasirox or deferriprone was not affected by chloroquine addition. The concentration of chloroquine used in these experiments effectively inhibited lysosomal hydrolysis, as shown previously by the inhibition of degradation of internalized EGF receptors.4 Chloroquine treatment for this time period did not affect cell viability as assayed by cell protein/plate. DFO-treated cells that had been incubated with chloroquine showed an accumulation of ferritin in lysosomes (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results show that DFO affects ferritin degradation by a route that is different from Fpn expression or the more membrane-permeable oral iron chelators.
DFO-induced ferritin loss is prevented by chloroquine treatment. HEK293T-Fpn cells were incubated with FAC (10μM Fe) for 24 hours followed by incubation in the absence or presence of 10μM ponasterone A, 100μM DFO, desferasirox, or deferriprone. Chloroquine was added at the final concentration of 100μM. After 10 hours, cells were harvested. The ferritin content was determined by ELISA. Error bars represent SD from 3 different experiments.
DFO-induced ferritin loss is prevented by chloroquine treatment. HEK293T-Fpn cells were incubated with FAC (10μM Fe) for 24 hours followed by incubation in the absence or presence of 10μM ponasterone A, 100μM DFO, desferasirox, or deferriprone. Chloroquine was added at the final concentration of 100μM. After 10 hours, cells were harvested. The ferritin content was determined by ELISA. Error bars represent SD from 3 different experiments.
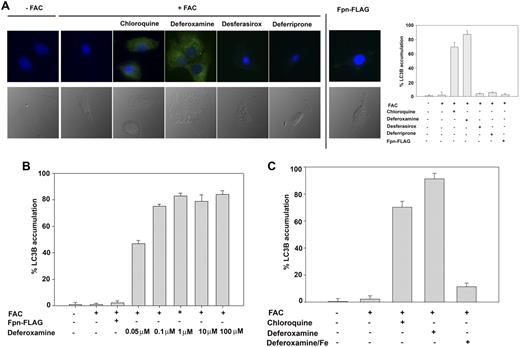
To determine how ferritin gains entry into the lysosome, we focused on autophagy. Autophagy is one of the few mechanisms known by which cytosolic proteins gain access to the lysosomal lumen.9 We tested the hypothesis that DFO induces autophagy leading to ferritin entry into the lysosomal lumen. To assess autophagy, we examined the expression and distribution of the autophagic marker LC3. LC3, a mammalian homolog of yeast Atg8, is cleaved during autophagy to generate LC3B, which is bound to autophagosomes, and the punctate accumulation of LC3B is a marker for autophagy.10 In control cells, there was little accumulation of LC3B but addition of chloroquine induced autophagy and led to the presence of punctate LC3B as shown by immunostaining (Figure 2A). Addition of DFO to cells induced the appearance of punctate LC3B but addition of desferasirox or deferriprone did not. Induction of Fpn did not result in LC3B accumulation. DFO-induced autophagy might be due to the concentration of DFO used but a clinically relevant steady-state concentration of DFO is within the range of 10 to 28μM.11-13 Autophagy, however, was still induced at concentrations that were 200-fold lower (Figure 2B). The ability of all 3 iron chelators to induce ferritin degradation was dependent on their iron-chelating activity. Addition of iron-saturated chelators to cells did not result in ferritin degradation and in the case of DFO, iron-saturated DFO did not induce autophagy (Figure 2C). These results indicate it is the iron-chelating activity of the chelators that is required for ferritin degradation.
DFO induces autophagy. (A) HeLa cells transfected with or without pCMV-Fpn-FLAG were incubated with FAC (10μM Fe) for 24 hours followed by incubation for 10 hours in the absence or presence of 10μM ponasterone A, 100μM DFO, desferasirox, or deferriprone. Cells were incubated with or without chloroquine for 5 hours. Cells were fixed and processed for immunofluorescence using mouse anti-LC3 antibody (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). The number of LC3B-positive cells was quantified (right panel) and error bars represent the analysis of greater than 5 fields per sample (at least 100 cells). Error bars represent SD from 3 different experiments. (B) HeLa cells expressing Fpn-FLAG were incubated with or without FAC (10μM) for 18 hours in the presence or absence of DFO at different concentrations (0.05, 0.1, 1, 10, 100μM). Cells were fixed and processed for immunofluorescence as in panel A. (C) HeLa cells were incubated with or without FAC (10μM) for 18 hours in the presence or absence of DFO (100μM) or DFO previously saturated with iron. Cells were incubated with or without chloroquine for 5 hours. Cells were fixed, processed for immunofluorescence, and quantified as in panel A.
DFO induces autophagy. (A) HeLa cells transfected with or without pCMV-Fpn-FLAG were incubated with FAC (10μM Fe) for 24 hours followed by incubation for 10 hours in the absence or presence of 10μM ponasterone A, 100μM DFO, desferasirox, or deferriprone. Cells were incubated with or without chloroquine for 5 hours. Cells were fixed and processed for immunofluorescence using mouse anti-LC3 antibody (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). The number of LC3B-positive cells was quantified (right panel) and error bars represent the analysis of greater than 5 fields per sample (at least 100 cells). Error bars represent SD from 3 different experiments. (B) HeLa cells expressing Fpn-FLAG were incubated with or without FAC (10μM) for 18 hours in the presence or absence of DFO at different concentrations (0.05, 0.1, 1, 10, 100μM). Cells were fixed and processed for immunofluorescence as in panel A. (C) HeLa cells were incubated with or without FAC (10μM) for 18 hours in the presence or absence of DFO (100μM) or DFO previously saturated with iron. Cells were incubated with or without chloroquine for 5 hours. Cells were fixed, processed for immunofluorescence, and quantified as in panel A.
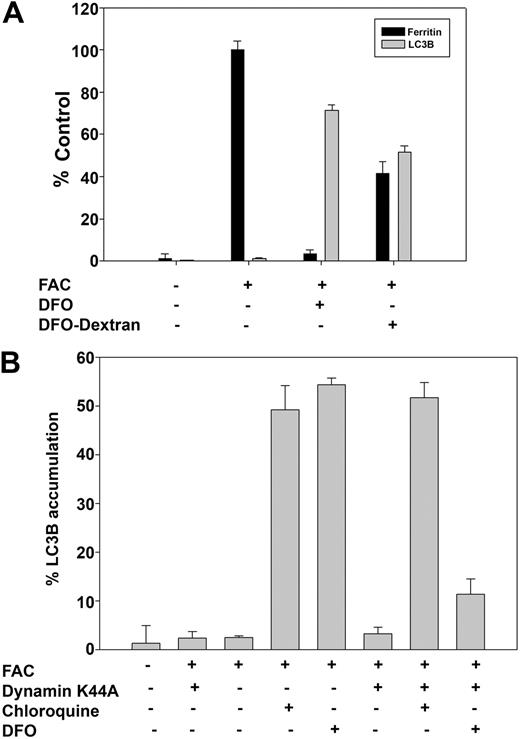
The inability of orally administrated DFO to be absorbed sparked a search for oral iron chelators. DFO is poorly membrane permeable, enters cells by endocytosis, and localizes to lysosomes and endosomes.14 Two approaches were taken to determine whether DFO is active within the endocytic apparatus. Cells were incubated with DFO-dextran conjugates to ensure that the chelator was in a membrane-impermeable form and ferritin loss and autophagy were assayed. DFO-dextran was able to induce both ferritin loss and LC3B accumulation (Figure 3A). We examined the ability of DFO to induce ferritin loss in cells in which endocytosis was inhibited. Cells were transfected with a plasmid expressing a dominant negative mutant (K44A) of dynamin, which blocks clathrin-mediated and caveolin-mediated endocytosis.15 In the absence of endocytosis, DFO did not induce autophagy, as shown by LC3B staining. Addition of chloroquine to dynamin K44A–transfected cells induced punctate LC3B staining, showing that it is the failure of DFO to gain entry into the lysosome that is responsible for the inhibition of DFO-induced autophagy (Figure 3B). These data indicate that DFO requires entry into the endocytic apparatus to effect ferritin degradation.
DFO in lysosomes induces autophagy. (A) HeLa cells were incubated with or without FAC (10μM) for 18 hours followed by incubation with or without DFO (100μM) or dextran-DFO for 16 hours. Cells were harvested for measurement of ferritin by ELISA or fixed and processed for immunofluorescence for LC3B as described in the Figure 2 legend. The amount of ferritin in FAC-loaded cells was taken as 100%. Error bars represent SD from 3 different experiments. (B) HeLa cells were transfected with or without dynamin K44A and incubated in the presence or absence of FAC (10μM) for 18 hours. DFO and chloroquine were added to a final concentration of 100μM for 6 hours. Cells were fixed and processed for immunofluorescence as in panel A. Percentage of LC3B accumulation was determined by analyzing 100 cells. Error bars represent SD from 3 different experiments.
DFO in lysosomes induces autophagy. (A) HeLa cells were incubated with or without FAC (10μM) for 18 hours followed by incubation with or without DFO (100μM) or dextran-DFO for 16 hours. Cells were harvested for measurement of ferritin by ELISA or fixed and processed for immunofluorescence for LC3B as described in the Figure 2 legend. The amount of ferritin in FAC-loaded cells was taken as 100%. Error bars represent SD from 3 different experiments. (B) HeLa cells were transfected with or without dynamin K44A and incubated in the presence or absence of FAC (10μM) for 18 hours. DFO and chloroquine were added to a final concentration of 100μM for 6 hours. Cells were fixed and processed for immunofluorescence as in panel A. Percentage of LC3B accumulation was determined by analyzing 100 cells. Error bars represent SD from 3 different experiments.
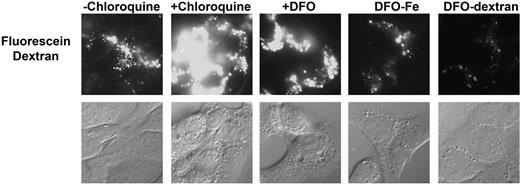
DFO contains a free amino group and resembles alkyl amines, which are lysomotrophic. It is unlikely that DFO can concentrate in lysosomes by diffusion because of the size and hydrophilicity of DFO, but it can enter lysosomes by endocytosis. The high pKa of the available amino group may alter lysosome pH. To test this possibility, cells were incubated with fluoresceinated dextran for 6 hours and then incubated in the absence of the dextran for 2 hours, permitting accumulation of fluorescein-dextran in lysosomes and late endosomes. Conjugation of DFO to dextran removes the free amino group, as the conjugation involves a Schiff base between the amino group on DFO and a hydroxyl ion on dextran. Cells were then incubated with chloroquine or DFO for 4 hours. Fluorescein is quenched by low pH and addition of chloroquine, by binding H+, increased lysosome pH resulting in an increase in fluorescein intensity (Figure 4). DFO also affected lysosomal pH, albeit to a lesser degree. Addition of DFO-dextran or iron-saturated DFO did not affect lysosomal pH. The fact that DFO-dextran induced lysosomal ferritin degradation but had little effect on lysosomal pH indicates that entry of ferritin into lysosomes is not the result of changes in lysosomal pH.
DFO does not induce pH changes in the lysosome. HeLa cells were incubated with fluorescein dextran for 6 hours and then incubated in the absence of dextran for 2 hours. Cells were then incubated with or without chloroquine, DFO, DFO saturated with iron (DFO-Fe), or DFO-dextran. Images were acquired on an epifluorescence microscope. The data are representative images from each condition found in 3 separate experiments.
DFO does not induce pH changes in the lysosome. HeLa cells were incubated with fluorescein dextran for 6 hours and then incubated in the absence of dextran for 2 hours. Cells were then incubated with or without chloroquine, DFO, DFO saturated with iron (DFO-Fe), or DFO-dextran. Images were acquired on an epifluorescence microscope. The data are representative images from each condition found in 3 separate experiments.
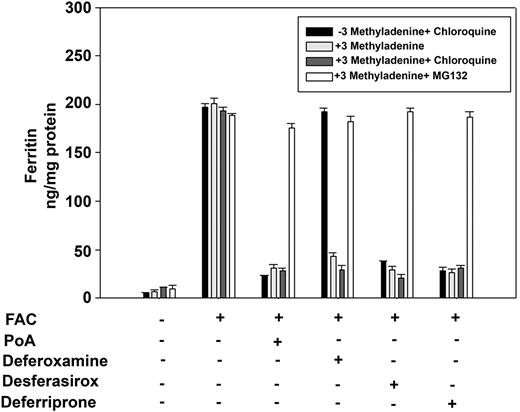
These results suggest that the presence of DFO within lysosomes can induce autophagy, which leads to the lysosomal accumulation and degradation of ferritin. We examined the effect of the autophagy inhibitor 3-methyladenine on DFO-induced ferritin loss. Addition of 3-methyladenine to iron-loaded cells did not affect ferritin levels and had no effect on ferritin loss in cells incubated with deferriprone or desferasirox or in cells expressing Fpn (Figure 5). Surprisingly, 3-methyladenine did not prevent DFO-induced loss of ferritin and chloroquine did not prevent ferritin degradation. DFO-induced ferritin degradation in 3-methyladenine–treated cells was prevented by the proteasomal inhibitor MG-132. These results demonstrate that the route for ferritin loss is not fixed and can occur either by lysosomal or proteosomal degradation. The fact that chloroquine-treated cells can degrade ferritin also indicates that chloroquine treatment (for the stated times) did not affect cell viability.
Inhibition of DFO-induced autophagy results in ferritin degradation in the proteasome. HEK293T-Fpn cells were incubated with FAC (10μM) for 24 hours followed by incubation in the presence or absence of 10μM ponasterone A (PoA) for 12 hours. Cells were incubated with or without 3-methyladenine for 8 hours in the presence or absence of 100μM chloroquine or 10μM MG132. FAC-loaded cells were also incubated with DFO, desferasirox, or deferriprone at the final concentration 100μM with or without 3-methyladenine for 8 hours in the presence or absence of 100μM chloroquine or 10μM MG132. Cells were harvested and ferritin content was determined by ELISA. Error bars represent SD from 4 different experiments.
Inhibition of DFO-induced autophagy results in ferritin degradation in the proteasome. HEK293T-Fpn cells were incubated with FAC (10μM) for 24 hours followed by incubation in the presence or absence of 10μM ponasterone A (PoA) for 12 hours. Cells were incubated with or without 3-methyladenine for 8 hours in the presence or absence of 100μM chloroquine or 10μM MG132. FAC-loaded cells were also incubated with DFO, desferasirox, or deferriprone at the final concentration 100μM with or without 3-methyladenine for 8 hours in the presence or absence of 100μM chloroquine or 10μM MG132. Cells were harvested and ferritin content was determined by ELISA. Error bars represent SD from 4 different experiments.
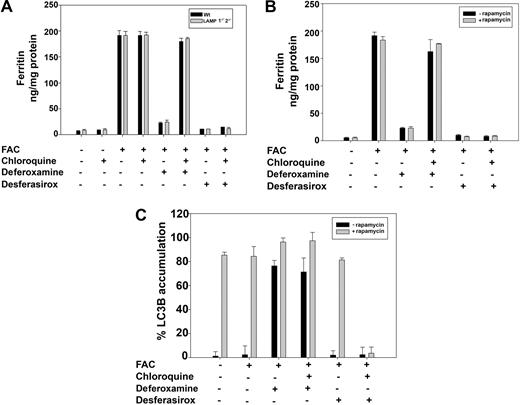
Autophagy provides a mechanism by which cytosolic proteins can gain access to the lumen of lysosomes. There are subdivisions of autophagy based on the selectivity of the cytosolic molecules or organelles engulfed. The most selective form of autophagy is referred to as chaperone-mediated autophagy (CMA), in which specific cytosolic proteins gain entry into lysosomes.16 CMA is dependent on an interaction between a motif on the cytosolic substrate and LAMP2, a lysosomal membrane protein. This motif occurs in approximately 30% of cytosolic proteins and there are sequences in ferritin that have similarity to the canonical motif. To determine whether DFO induces autophagy by CMA, we took advantage of cell lines derived from mice with targeted gene deletions in LAMP1 and LAMP2.17 Addition of iron to wild-type or LAMP1−/−2−/− fibroblasts resulted in ferritin accumulation (Figure 6A). Removal of iron-containing media and addition of iron chelators resulted in the loss of ferritin in all cell types. As expected, chloroquine inhibited ferritin degradation in DFO-treated cells but not in cells treated with desferasirox. In LAMP1−/−2−/− cells, chloroquine prevented DFO-induced loss of ferritin, implying that ferritin gained access to the lysosome but was not degraded. These results indicate that DFO does not induce ferritin entry into lysosomes by CMA.
Ferritin degradation is mTOR and LAMP 1-2 independent. (A) Wt and LAMP1−/−2−/−cells were incubated with or without FAC (10μM) for 24 hours. Cells were then incubated with 100μM DFO or desferasirox in the presence or absence of 100μM chloroquine for 8 hours. Cells were harvested and ferritin content was determined by ELISA. Error bars represent SD from 3 different experiments. (B) HeLa cells treated as in panel A were incubated in the presence or absence of 100nM rapamycin for 12 hours. Cells were harvested and ferritin content was determined by ELISA. (C) Cells as in panel B were stained for LC3B and the percentage of LC3B-positive cells was determined. Error bars represent SD from 3 different experiments.
Ferritin degradation is mTOR and LAMP 1-2 independent. (A) Wt and LAMP1−/−2−/−cells were incubated with or without FAC (10μM) for 24 hours. Cells were then incubated with 100μM DFO or desferasirox in the presence or absence of 100μM chloroquine for 8 hours. Cells were harvested and ferritin content was determined by ELISA. Error bars represent SD from 3 different experiments. (B) HeLa cells treated as in panel A were incubated in the presence or absence of 100nM rapamycin for 12 hours. Cells were harvested and ferritin content was determined by ELISA. (C) Cells as in panel B were stained for LC3B and the percentage of LC3B-positive cells was determined. Error bars represent SD from 3 different experiments.
A second mechanism of autophagy is macroautophagy, in which lysosomes nonselectively engulf cytosolic components.18 Some forms of macroautophagy can be activated by rapamycin, an inhibitor of the TOR pathway.19 Incubation of cells with rapamycin did not induce ferritin degradation nor did it prevent DFO-induced degradation of ferritin, whereas chloroquine treatment prevented DFO-induced degradation of ferritin (Figure 6B). These results indicate that entry of ferritin into lysosomes is sensitive to 3-methyladenine but does not occur by either TOR- or CMA-mediated autophagy. Rapamycin did not induce ferritin degradation but did lead to accumulation of LC3B (Figure 6C). This result indicates that the induction of ferritin entry into the lysosomes may not be the result of macroautophagy, but rather is a specific process that requires the presence of iron chelator activity in lysosomes.
Discussion
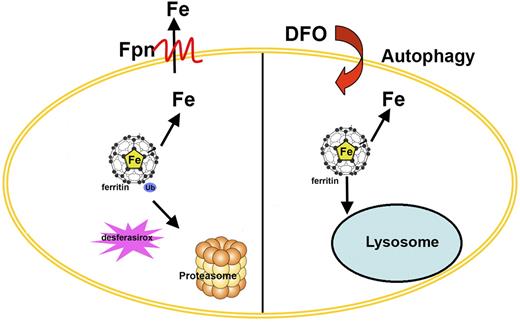
Retrieval of iron from ferritin provides a source of this essential metal during periods of scarcity. The mechanism of iron retrieval, however, has been unclear. Some studies have shown that ferritin is degraded in the lysosome and that the degradation is a prerequisite for iron release. The stimuli that induce ferritin entry into the lysosome are varied and include amino acid deprivation,20 Nesseria infection,21 or treatment with the iron chelator DFO.6 In contrast, expression of Fpn (the only known cell surface iron exporter) induces iron exit from ferritin and the resultant apoferritin is degraded in the proteasome.4 Other studies have yielded conflicting data on ferritin degradation. Treatment of cells with oxidizing agents such as H2O2 leads to ferritin degradation by the proteasome,22,23 whereas ferritin degradation induced by doxorubicin is inhibited by both proteasomal and lysosomal inhibitors.24 The studies presented here clarify the mechanisms of ferritin degradation (Figure 7). First, agents that lower cytosolic iron level leads to ferritin degradation by the proteasome. This conclusion is based on the observation that membrane-permeable iron chelators lead to ferritin degradation by the proteasome. In contrast, DFO, which is poorly permeable, leads to ferritin degradation in the lysosome. This effect of DFO is due to the localization of DFO in lysosomes as shown by the fact that DFO attached to dextran, which increases its membrane impermeability, also leads to ferritin degradation that is sensitive to chloroquine. Inhibition of endocytosis prevents DFO-induced ferritin degradation, supporting the importance of the lysosomal localization of DFO. DFO localization in lysosomes is correlated with an induction of autophagy. Iron-loaded DFO or addition of DFO when endocytosis is inhibited does not induce autophagy. In preliminary studies, we observed that addition of the impermeable iron chelator bathophenanthroline disulfonate to cells did not induce autophagy, suggesting that induction of autophagy might be specific for bacterial siderophores (data not shown).
Model of alternate routes for ferritin degradation. The model shows that ferritin degradation can occur by different routes. Ferroportin (Fpn) or oral iron chelator (desferasirox)–mediated ferritin degradation occurs by the proteasome. In contrast, ferritin degradation induced by DFO occurs in the lysosome.
Model of alternate routes for ferritin degradation. The model shows that ferritin degradation can occur by different routes. Ferroportin (Fpn) or oral iron chelator (desferasirox)–mediated ferritin degradation occurs by the proteasome. In contrast, ferritin degradation induced by DFO occurs in the lysosome.
Autophagy provides a mechanism by which cytosolic proteins gain access to the lumen of lysosomes. Autophagy can be induced by inhibition of the TOR pathway.19 Rapamycin, which inhibits the TOR pathway, can induce macroautophagy but does not induce ferritin degradation. Inhibition of lysosomal proteolysis by chloroquine induces autophagy but does not prevent the proteosomal degradation of ferritin. If autophagy of ferritin led to ferritin sequestration within lysosomes that unable to degrade ferritin, then ferritin would not be available for proteasomal degradation. Our results suggest that entry of ferritin into lysosomes is highly specific and not merely a consequence of generalized engulfment of cytosolic compartments by lysosomes. Some form of autophagy, however, is required for ferritin degradation because the lysosomal degradation of ferritin can be inhibited by 3-methyladenine, an inhibitor of PI kinase activity.25
The use of 3-methyladenine reveals that there is plasticity in the route by which ferritin is degraded. We previously demonstrated that ferroportin induces ferritin degradation by the proteasome.4 In cells that have a temperature-sensitive mutation in the E1 ubiquitin ligase, incubation of cells at the restrictive temperature blocked the ferroportin-mediated degradation of ferritin. Incubation of those cells with DFO led to the degradation of ferritin in the lysosome, as shown by the ability of chloroquine to block ferritin loss. Addition of 3-methyladenine prevented ferritin degradation in DFO-treated cells at the restrictive temperature. Here we demonstrate that 3-methyladenine blocks ferritin degradation by the lysosome and leads to ferritin degradation by the proteasome. In aggregate, our results show that ferritin can be degraded either by the lysosome or the proteasome. Our studies suggest that the iron-chelating activity of lysosomal DFO can induce ferritin degradation even when entry of ferritin into the lysosome is prevented. The finding that DFO-dextran has the same effect as DFO supports the view that it is the presence of iron-chelating activity in lysosomes that induces ferritin degradation. This result suggests that there is some transport system/channel that permits lysosomal iron to communicate with cytosolic iron. Previously, we demonstrated that incubation of cells with cationic ferritin induced the synthesis of endogenous ferritin.26 Cationic ferritin is endocytosed and directed to the lysosome where it is degraded. Iron released within the lysosome then accesses the cytosol where it induces the synthesis of endogenous ferritin. This result indicates that iron can exit the lysosome. Recent studies have suggested candidate transporters that mediate lysosome to cytosol iron movement.27,28 The finding that iron-chelation activity within the lysosome can induce the degradation of cytosolic ferritin provides evidence for a channel/transporter that might move iron in both directions, from lysosome to cytosol and from cytosol to lysosome. Recent studies have suggested candidates for transporters that mediate iron exit from the lysosome.27,28 The number of transporters and the mechanism of transport remain to be clarified. It is possible that such transporters can function bidirectionally depending on iron gradients. It is also possible that transporters exist that mediate iron entry into the lysosome. A precedent for such transporters exists in fungi and plants in which there are separate transporters for iron entry into vacuoles (the plant and fungi equivalent of lysosomes) and for iron exit from vacuoles.29 Our studies used cultured fibroblasts and epithelial-like cells (HeLa and HEK293). We recognize that chelators may have different effects on differentiated cell types such as hepatocytes and macrophages. Studies on such cell types would be required to extend the generality of these results.
Recent studies reported that combinations of DFO with either of the oral iron chelators may provide better management of patients with iron overload disease than does a single chelator. It has been suggested that the combination of 2 chelators provides an “iron shuttle” in which the permeable iron chelators access compartments inaccessible to the less permeable DFO. Iron bound to the permeable chelator is transferred to the impermeable chelators, permitting the permeable iron chelators to rebind iron.30 We speculate that DFO, by accumulating in lysosomes, can promote iron release by 2 additional effects. First, DFO has access to iron accumulated in lysosomes, most notably hemosiderin deposits. The concentration of DFO in lysosomes in conjunction with its high iron avidity may permit DFO to mobilize hemosiderin iron deposits. Second, by inducing lysosomal catabolism of ferritin, DFO enhances iron depletion by the permeable iron chelators.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Prem Ponka (McGill University) for the iron chelators deferriprone and desferasirox, Dr Paul Saftig (University of Kiel) for the wild-type and LAMP deletion mouse cell lines, and Dr J. P. Kushner for his help in editing this paper.
This work was supported by National Institutes of Health (NIH) grants DK030534 and DK070947 (J.K.).
National Institutes of Health
Authorship
Contribution: I.D.D. and D.M.W. performed experiments, analyzed data, and wrote the paper; and J.K. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerry Kaplan, Department of Pathology, University of Utah School of Medicine, 50 N Medical Dr, Salt Lake City, UT 84132; e-mail: jerry.kaplan@path.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal