Abstract
Atypical hemolytic uremic syndrome (aHUS) is associated with complement system dysregulation, and more than 25% of pediatric aHUS cases are linked to mutations in complement factor H (CFH) or CFH autoantibodies. The observation of thrombocytopenia and platelet-rich thrombi in the glomerular microvasculature indicates that platelets are intimately involved in aHUS pathogenesis. It has been reported that a releasable pool of platelet CFH originates from α-granules. We observed that platelet CFH can arise from endogenous synthesis in megakaryocytes and that platelets constitutively lacking α-granules contain CFH. Electron and high-resolution laser fluorescence confocal microscopy revealed that CFH was present throughout the cytoplasm and on the surface of normal resting platelets with no evident concentration in α-granules, lysosomes, or dense granules. Therapeutic plasma transfusion in a CFH-null aHUS patient revealed that circulating platelets take up CFH with similar persistence of CFH in platelets and plasma in vivo. Washed normal platelets were also observed to take up labeled CFH in vitro. Exposure of washed normal platelets to plasma of an aHUS patient with CFH autoantibodies produced partial platelet aggregation or agglutination, which was prevented by preincubation of platelets with purified CFH. This CFH-dependent response did not involve P-selectin mobilization, indicating a complement-induced platelet response distinct from α-granule secretion.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a severe kidney disease that mainly affects children. Treatment options are few and mostly empirical, and risks remain significant for progression to end-stage kidney disease and death.1 aHUS pathogenesis is associated with injury to vascular endothelial cells and the formation of platelet-rich thrombi, which occlude glomerular capillary loops and compromise renal function.2,3 Genetic evidence indicates that the key initial determinants of aHUS are defects in the regulation of the complement system, specifically the constitutively active alternative pathway.4 Mutations in the complement regulators factor I,5,6 membrane cofactor protein (MCP/CD46),7,8 factor B (gain-of-function mutation),9 factor H–related proteins 1 and 3,10 and complement factor H (CFH)11,12 have been implicated in aHUS pathogenesis, as have CFH autoantibodies.13,14 CFH mutations and autoantibodies both compromise the ability of CFH to inhibit complement activation on the surface of vascular endothelial cells, resulting in cell injury and exposure of the extracellular matrix, a well-established physiologic trigger for platelet activation. Thus, endothelium injury-induced platelet activation has been proposed as the main cause of aHUS-associated thrombocytopenia and thrombus formation.4,15
There is also evidence that interaction between platelets and the alternative complement pathway plays a significant role in aHUS pathogenesis.16 Platelets represent a unique point of convergence between the complement and coagulation systems. Complement cascade components are present on the platelet surface, and platelet activation can induce complement activation,17 whereas complement activation on the surface of platelets can trigger platelet activation.16 Consequently, factors that compromise complement regulation on platelets may influence platelet activity and vice versa.18
We report the results of studies aimed at characterizing the interactions of platelets and CFH to better understand their involvement in aHUS pathogenesis. It has been claimed that platelets contain a releasable pool of CFH localized to α-granules19 and that CFH probably binds to glycoprotein (GP) IIb/IIIa (αIIbβ3 integrin) surface receptors via the C-terminus.20 Yet the source of platelet-associated CFH (ie, platelet-CFH) remains unclear. Our studies revealed that CFH is not localized to platelet α-granules; rather, it is observed throughout the cytoplasm and on the surface. We also show that CFH is endogenously synthesized by megakaryocytes and that CFH can be taken up by CFH-deficient platelets in vivo and by normal platelets in vitro. Lastly, we demonstrate that plasma from an aHUS patient with CFH autoantibodies can induce a CFH-dependent platelet aggregation or agglutination response that does not result in α-granule secretion. These findings suggest an important role for CFH in modulating platelet structure and function, which is probably intimately involved in aHUS pathogenesis.
Methods
Patients
This study was performed according to the guidelines of the Research Ethics Board at The Hospital for Sick Children in Toronto and the Children's Hospital of the University of Cologne. Informed consent was provided according to the Declaration of Helsinki.
Blood samples were collected from 2 reported aHUS patients. Patient 1 is a 6.5-year-old boy with aHUS resulting from CFH deficiency caused by a point mutation in the CFH gene (T2770A), resulting in a premature stop codon (Y899Stop).21 Full-length plasma CFH was undetectable, whereas the alternate splice variant factor H–like protein 1 (FHL-1) was present in normal amounts.21 Plasma transfusions were successful in the acute phase and, when provided in 14-day cycles, prevented further relapses.21 Patient 2 is a 15-year-old girl with aHUS. Although plasma CFH was within normal limits, there was complete absence of CFH-related proteins 1 and 3 caused by a homozygous genomic deletion,10 and CFH autoantibodies14 were detected. Treatment with plasma exchange and steroids was successful during acute presentation, and subsequent biweekly plasma transfusions kept her relapse-free to date.
Preparation of plasmas, washed platelets, and cell lysates
Blood samples were collected into either 3.2% citrate (some controls and all aHUS patients) or acid citrate dextrose (ACD). Platelet-rich plasma (PRP) was prepared via centrifugation (180g, 15 minutes) of blood samples, and platelets were recovered from PRP via centrifugation (1000g, 10 minutes); platelet-poor plasma was retained after further centrifugation at 14 000g for 10 minutes. Normal and aHUS patient platelet-poor plasma used for platelet aggregation experiments was passed through a 0.2-μm filter. Washed platelets were prepared via resuspension in phosphate-buffered saline (PBS) buffer adjusted to pH 6.1 with ACD, centrifugation, and resuspension (double-washed platelets were subjected to 2 cycles, triple-washed to 3). Normal and arthrogryposis, renal tubular dysfunction, and cholestasis (ARC) platelet lysates were made from double-washed platelets resuspended at 109 platelets/mL with PBS plus 2× protease inhibitor (Roche Complete ethylenediaminetetraacetic acid-free; Roche Diagnostics) using Triton X-100 (0.5%) as previously described.22 Megakaryocyte lysates were from cells grown in serum-free suspension culture.22 Plasma and lysate samples were stored at −70°C before use.
Immunoblot analysis
Cell lysate and plasma protein samples were separated via 10% nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis before transfer to nitrocellulose membranes. Immunoblots were initially probed with a rabbit polyclonal antibody specific for the amino terminal of CFH (SCRs1-4; provided by P.F.Z.), which also allowed for the detection of FHL-1 because it shares the same coding region. Immunoblots were subsequently probed with one or more of: rabbit polyclonal anti–platelet factor 4 (Millipore/Chemicon), mouse anti–thrombospondin-1 (TSP1; Thermo Scientific/Medicorp), mouse anti–β-actin (Sigma-Aldrich Canada), goat anti–human factor XII (FXII; Cedarlane Laboratories). Secondary antibodies used were HRP-conjugated donkey anti–rabbit, –mouse, or –goat (Jackson ImmunoResearch Laboratories). Imaging was conducted via Western Lightning ECL (PerkinElmer Health Sciences) onto x-ray films scanned at 600 dpi. Blot images were assembled for publication using Adobe Photoshop (CS3).
Immunogold transmission electron microscopy
Citrate anticoagulated blood was fixed for 30 minutes with an equal volume of PBS plus 4% paraformaldehyde (PFA; Electron Microscopy Sciences) and centrifuged to recover PRP, which was mixed with an equal volume of PBS plus 4% PFA for 30 minutes before pelleting platelets by centrifugation. Platelets were subjected to dehydration in a graded series of ethanol concentrations (50%, 70%, 90%, and 100%; 2 × 15 minutes each) at 4°C. Platelets were stored overnight in 100% ethanol mixed with Lowicryl resin (1:1) at 4°C. Resin samples were embedded in tightly sealed BEEM capsules, polymerized for 3 days under ultraviolet light at −20°C. Thin sections mounted on Formvar-coated nickel grids were placed on droplets of 1% glycine/PBS, followed by a blocking step with PBS plus 5% skim milk, incubated in primary rabbit anti-CFH antibody (SCRs1-4; 1:20) for 60 minutes, washed with PBS plus 1% bovine serum albumin (BSA; MP Biomedicals), incubated in goat anti–rabbit secondary antibody conjugated with 10nM colloidal gold (GE Healthcare) in PBS plus 1% BSA for 60 minutes, washed with PBS, fixed with PBS plus 2.5% glutaraldehyde for 15 minutes, rinsed with water, and dried. Immunostained thin sections were counterstained with 2% uranyl acetate and lead citrate and examined with a JEOL JEM-1011 electron microscope at 80 kV. Digital images were captured with a side-mounted Advantage HR CCD camera (Advanced Microscopy Techniques), saved in TIFF format, and trimmed for publication using Adobe Photoshop (CS3).
Laser fluorescence spinning disk confocal microscopy
Preparation of platelets for immunofluorescence analysis was conducted via a modification of the method of Sehgal and Storrie.23 PRP was mixed with an equal volume of PBS plus 8% PFA and incubated for 15 minutes at room temperature. Platelets were recovered via centrifugation, washed twice in PBS/ACD, and resuspended at approximately 200 × 109 platelets/L in Tyrode buffer, pH 6.5, plus 0.1% BSA, 1mM CaCl2, 1mM MgCl2, and 3μM prostaglandin E1 (Sigma-Aldrich). An equal volume of PBS plus 8% PFA was added to the platelet suspension; and after incubation for 15 to 30 minutes at room temperature, 100-μL aliquots were placed on 25-mm circular glass coverslips in tissue-culture dishes and incubated for 90 minutes floating in a 37°C water bath. Platelet spreads were washed with PBS, permeabilized with 0.5% Triton X-100 for 5 minutes, washed, and blocked for a minimum of 60 minutes with PBS plus 1% BSA and 2% normal goat serum. For CFH colocalization studies, cells were exposed to rabbit polyclonal anti-CFH antibody (SCRs19-20; 1:200; provided by P.F.Z.) in combination with mouse monoclonal antibody specific for one of: α-tubulin (1:2000; Sigma-Aldrich), fibrinogen (1:100; BD Biosciences PharMingen), von Willebrand factor (VWF, 1:25; Dako North America), CD-61 (1:100; Immunotech), CD-63 (1:100; BD Biosciences PharMingen), β-actin (1:1000;Sigma-Aldrich), or LAMP-1 (1:25; clone H4A3, Developmental Studies Hybridoma Bank, University of Iowa). After washing, platelet preparations were incubated for approximately 60 minutes with fluorescent-tagged donkey antibodies specific for rabbit (Alexa Fluor 488 conjugated; Invitrogen) and mouse (Cy3 or Alexa Fluor 568 conjugated; Invitrogen) IgG, washed, and the coverslips mounted on slides with fluorescent mounting medium (Dako North America). Preparations were imaged with a 100× magnification wide-field TIRF objective (1.4 numerical aperture) on a Leica DMIRE2 inverted fluorescence microscope equipped with a Leica focus drive, 1.5× magnification lens (Spectral Applied Research), separate diode-pumped solid state laser lines and a spinning disk confocal scan head (Quorum Technologies). Z-stack images (0.25-μm stepping) of flat resting platelets (integrity indicated by the presence of an intact tubulin ring) were acquired via a Hamamatsu Back-Thinned EM-CCD camera at 512 × 512 pixels using the Improvision Volocity acquisition software module, and subsequently deconvolved, rendered, and subjected to single-cell colocalization analysis via the appropriate Volocity software (Version 3) modules running on an Apple Mac Pro. Snapshots were trimmed, labeled, and arranged using Adobe Photoshop and Illustrator (CS3).
Platelet uptake of labeled CFH
Purified human CFH (CSL Behring) was conjugated with Alexa Fluor 488 via reaction with Alexa Fluor 488 5-SDP ester (Invitrogen) following the manufacturer's protocol. The resulting A488-CFH was determined to have approximately 4 fluorescent tags per molecule. Triple-washed normal platelets (see “Preparation of plasmas, washed platelets, and cell lysates”) were resuspended at 400 × 109 platelets/L in Tyrode buffer, pH 6.5, plus 0.1% BSA, 1mM CaCl2, 1mM MgCl2, and incubated at 37°C in buffer alone or with 100 μg/mL A488-CFH. Aliquots were taken after the start of incubation at 30, 60, and 120 minutes, mixed with an equal volume of PBS plus 8% PFA, fixed at room temperature for 10 minutes, washed with PBS/ACD, and used to prepare platelet spreads (see “Laser fluorescence spinning disk confocal microscopy”). Platelet preparations were immunostained and examined via laser fluorescence confocal microscopy using rabbit polyclonal antibody specific for platelet myosin IIA (1:500; Biomedical Technologies Inc) and mouse monoclonal antibody specific for α-tubulin (Sigma-Aldrich).
Platelet aggregometry
Donor blood was collected into ACD (1:6), and washed platelets (see “Preparation of plasmas, washed platelets, and cell lysates”) resuspended at 200 × 109 platelets/L in Tyrode buffer, pH 7.4, plus glucose, 2mM Ca2+, 2mM Mn2+ without or with 200 μg/mL purified human CFH (CSL Behring). Plasma samples were from citrated blood, filtered, and used fresh (autologous plasma), or after having been stored at −70°C (ABO-matched patient and normal donor plasma). Platelet aggregometry was performed with a Chronolog Model 700 optical aggregometer (Chronolog Corporation) in mini-cuvettes at 37°C with 400 rpm stirring. A 150-μL aliquot of plasma was added to an equal volume of platelet suspension at the start of each run, resulting in a final platelet concentration of 100 × 109/L and a final CFH concentration of either 0 or 100 μg/mL.
Flow cytometry
Experiments were conducted to assess the effects of plasma on platelets under both dynamic and static conditions. In the former, platelets were sampled from aggregometry cuvettes at the end of a run (see “Platelet aggregometry”), fixed with 4% PFA, and stained with mouse anti-CD62P-PE (BD Biosciences PharMingen). In the static experiment, 20-μL aliquots of platelet suspension (see “Preparation of plasmas, washed platelets, and cell lysates”) were incubated with equal amounts of plasma for 20 minutes, fixed in 4% PFA, and stained with mouse anti–CD62-PE. Samples were assayed for surface CD62P (P-selectin) expression using a FACSCalibur system (BD Biosciences).
Results
CFH in human megakaryocytes and platelets
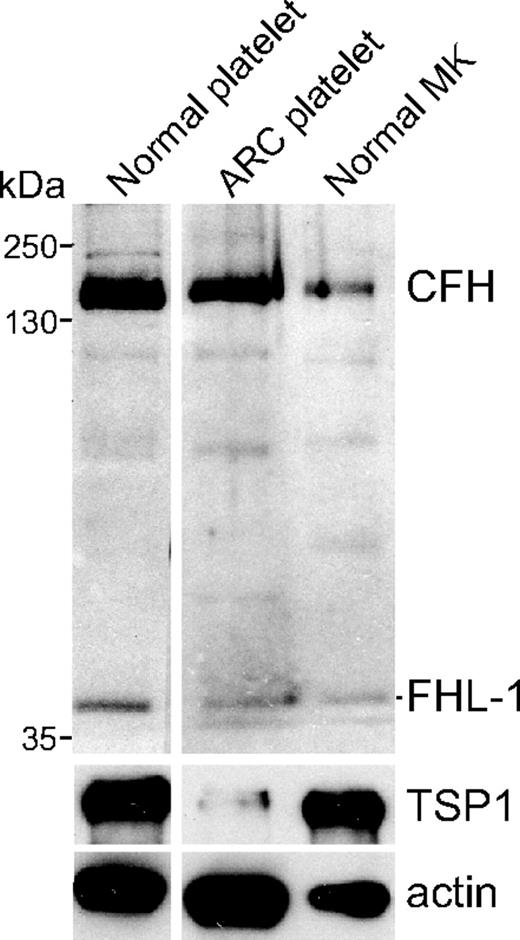
The presence of CFH in human megakaryocytes, platelets, and platelet α-granules was examined via immunoblot analysis of proteins derived from whole-cell lysates of megakaryocytes grown in serum-free suspension cultures and platelets from normal donors and an ARC syndrome patient lacking α-granules.22 Probing with a rabbit polyclonal antibody (SCRs1-4) for CFH and FHL-1 detected both proteins in lysates from normal megakaryocytes and platelets, and from α-granule–deficient ARC platelets (Figure 1). The absence of α-granules and constituent proteins in ARC platelets is indicated by the absence of the α-granule protein TSP1 (Figure 1 lane 2). Thus, CFH and FHL-1 are synthesized by normal human megakaryocytes, and both proteins are present in circulating platelets in the absence of α-granules.
CFH and FHL-1 content in normal platelets, platelets lacking α-granules, and human megakaryocytes. Immunoblot of platelet and megakaryocyte whole-cell lysates consisting of washed platelets (107 platelets/lane) from a normal person (Normal platelet), an ARC syndrome patient (ARC platelet), and serum-free cultured megakaryocytes (Normal MK). The blot was initially probed with a rabbit polyclonal antibody capable of detecting CFH and FHL-1 and subsequently with antibodies specific for TSP1 and β-actin, used as α-granule content and protein-loading indicators, respectively. Both CFH and FHL-1 are present in normal megakaryocytes and platelets, and also in ARC platelets lacking α-granules (near absence of the α-granule protein TSP1).
CFH and FHL-1 content in normal platelets, platelets lacking α-granules, and human megakaryocytes. Immunoblot of platelet and megakaryocyte whole-cell lysates consisting of washed platelets (107 platelets/lane) from a normal person (Normal platelet), an ARC syndrome patient (ARC platelet), and serum-free cultured megakaryocytes (Normal MK). The blot was initially probed with a rabbit polyclonal antibody capable of detecting CFH and FHL-1 and subsequently with antibodies specific for TSP1 and β-actin, used as α-granule content and protein-loading indicators, respectively. Both CFH and FHL-1 are present in normal megakaryocytes and platelets, and also in ARC platelets lacking α-granules (near absence of the α-granule protein TSP1).
Intracellular localization of CFH in platelets
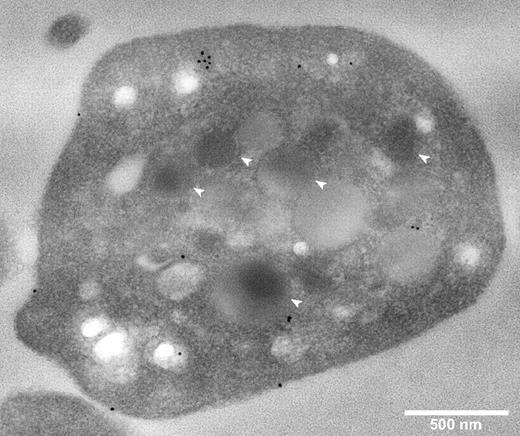
The intracellular localization of CFH in fixed permeabilized platelets was determined by immunostaining and imaging of sections via transmission electron microscopy (TEM), and of whole cells by laser fluorescence spinning disk confocal microscopy. TEM of immunogold-labeled sections (Figure 2) showed CFH-labeled protein distributed around the cell surface and throughout the cytoplasm, with no evident specific association with α-granules or other organelles. A rabbit polyclonal anti–human CFH (SCRs19-20) antibody was used for these experiments and those described in Figure 3; an antibody specific for CFH (SCRs1-4) recognizing the N-terminal region gave the same results (data not shown).
Platelet CFH is distributed throughout the cell and not preferentially localized to α-granules. Thin-section TEMs of representative normal platelets immunogold labeled for CFH (original magnification ×40 000). White bar represents 500 nm; white arrows, α-granules. Primary anti-CFH rabbit polyclonal antibody was detected by immunogold-tagged (10 nm) secondary antibody (black spots). Immunogold-CFH was distributed throughout the cytoplasm with some on the surface but none in recognizable α-granules (compare white arrows with black spots).
Platelet CFH is distributed throughout the cell and not preferentially localized to α-granules. Thin-section TEMs of representative normal platelets immunogold labeled for CFH (original magnification ×40 000). White bar represents 500 nm; white arrows, α-granules. Primary anti-CFH rabbit polyclonal antibody was detected by immunogold-tagged (10 nm) secondary antibody (black spots). Immunogold-CFH was distributed throughout the cytoplasm with some on the surface but none in recognizable α-granules (compare white arrows with black spots).
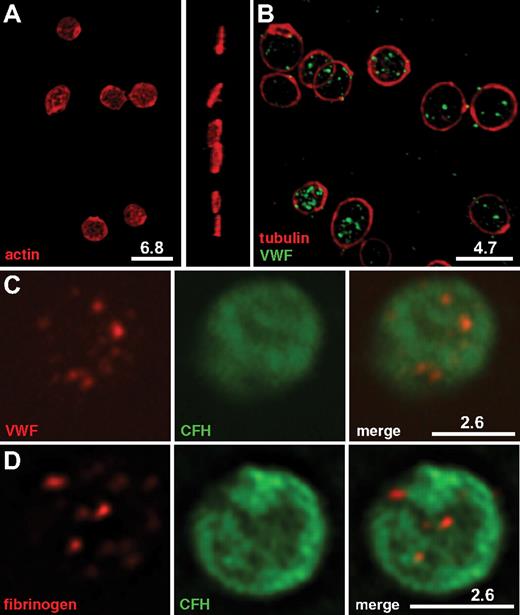
Immunofluorescence imaging and localization of CFH within normal platelets. Washed normal human platelets were fixed, permeabilized, and incubated with pairs of mouse monoclonal and rabbit polyclonal antibodies, followed by labeling with secondary antibodies tagged with Alexa Fluor 568 or Cy3 (red), or Alexa Fluor 488 (green). White bars with the corresponding microns are indicated. All images were deconvolved. (A-B) Rendered 3-dimensional images. (C-D) Representative midplatelet Z-slices. (A) Staining of intact platelets for actin reveals the plate-like shape of resting cells in both top (left) and perpendicular (right) views, whereas (B) tubulin-staining microtubule rings (red) surrounding VWF-containing α-granules (green) confirm the nonactivated state. (C-D) CFH labeled with Alexa Fluor 488 (green) is shown in the middle panels; right panels, the corresponding merged left and middle panels. Costaining of CFH (green) with VWF (C, red) or fibrinogen (D, red) confirms the presence of CFH throughout platelets and does not indicate specific colocalization with either α-granule marker. Results of whole-platelet colocalization analysis are presented in Table 1.
Immunofluorescence imaging and localization of CFH within normal platelets. Washed normal human platelets were fixed, permeabilized, and incubated with pairs of mouse monoclonal and rabbit polyclonal antibodies, followed by labeling with secondary antibodies tagged with Alexa Fluor 568 or Cy3 (red), or Alexa Fluor 488 (green). White bars with the corresponding microns are indicated. All images were deconvolved. (A-B) Rendered 3-dimensional images. (C-D) Representative midplatelet Z-slices. (A) Staining of intact platelets for actin reveals the plate-like shape of resting cells in both top (left) and perpendicular (right) views, whereas (B) tubulin-staining microtubule rings (red) surrounding VWF-containing α-granules (green) confirm the nonactivated state. (C-D) CFH labeled with Alexa Fluor 488 (green) is shown in the middle panels; right panels, the corresponding merged left and middle panels. Costaining of CFH (green) with VWF (C, red) or fibrinogen (D, red) confirms the presence of CFH throughout platelets and does not indicate specific colocalization with either α-granule marker. Results of whole-platelet colocalization analysis are presented in Table 1.
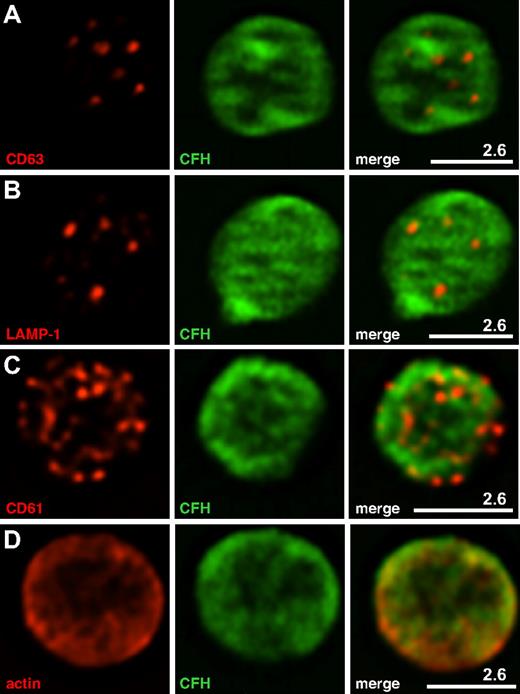
The whole-cell distribution of CFH was examined in fixed, permeabilized platelets costained for CFH and other proteins having known intracellular distributions (Figures 3–4). Cells in an unperturbed, resting state were examined as illustrated in Figure 3A where β-actin staining outlines the plate-like shape of cells viewed from the top and side, and Figure 3B where tubulin rings (red) delineate quiescent platelets with intact VWF-containing α-granules (green). These granules were consistent in size and distribution with published images.23 Because previous reports suggested that CFH is localized to platelet α-granules, we first assessed its distribution relative to the known granule-associated proteins VWF (Figure 3C) and fibrinogen (Figure 3D), which are present in 2 distinct populations of α-granules,23 CD63 (Figure 4A), which is present in both dense (δ-) granules and lysosomes, and the lysosomal protein LAMP-1 (Figure 4B). The results show that, unlike these proteins, CFH is not concentrated in granules but rather appears to be present throughout the cytoplasm and possibly on the cell surface (as seen in TEM images; Figure 2). The presence of CFH in these locations is supported by the overlap observed in cells costained for CD61 (GPIIIa, β3 integrin; Figure 4C), found on the cell surface and in the cytoplasm, and the cytoplasmic protein β-actin (Figure 4D). Unpermeabilized platelets showed markedly less CFH staining (data not shown), indicating that much of the protein is inside (washed) cells. These visual impressions were confirmed by the results of whole-cell protein colocalization analysis of representative images (Table 1). Mean values obtained for the Pearson correlation coefficient indicate significant colocalization of CFH with β-actin and CD61, but not with vesicle-associated proteins. The values obtained for the Manders overlap coefficient confirm the strong colocalization of CFH with β-actin and a weaker association with CD61 (for which the value of the overlap coefficient falls below the arbitrary significance limit of 0.7),24 which is nevertheless stronger than the association with any of the other proteins examined. Taken together, both TEM and laser fluorescence confocal microscopy studies indicated that CFH is distributed throughout platelets and to some extent on the cell surface, and is not concentrated in or confined to α-granules or other secretory vesicles.
Immunofluorescence localization of CFH within normal platelets. Washed normal human platelets were prepared and imaged as described in Figure 3. CFH labeled with Alexa Fluor 488 (green) is shown in the middle panels; right panels, merged left and middle panels. White bars with the corresponding microns are indicated. (A-D) Representative midplatelet Z-slices. (A) The δ-granule and lysosome protein CD63 does not show specific colocalization with CFH, nor does the lysosomal protein LAMP-1 (B). (C) Partial colocalization of CFH is observed with the platelet surface protein CD61 (β3 integrin), and (D) strong colocalization is apparent with cytoplasmic actin. Results of whole-cell colocalization analysis are presented in Table 1.
Immunofluorescence localization of CFH within normal platelets. Washed normal human platelets were prepared and imaged as described in Figure 3. CFH labeled with Alexa Fluor 488 (green) is shown in the middle panels; right panels, merged left and middle panels. White bars with the corresponding microns are indicated. (A-D) Representative midplatelet Z-slices. (A) The δ-granule and lysosome protein CD63 does not show specific colocalization with CFH, nor does the lysosomal protein LAMP-1 (B). (C) Partial colocalization of CFH is observed with the platelet surface protein CD61 (β3 integrin), and (D) strong colocalization is apparent with cytoplasmic actin. Results of whole-cell colocalization analysis are presented in Table 1.
Quantitative whole-cell colocalization analysis of CFH in platelets costained with organelle, surface, and cytoplasmic proteins
| Protein (red*) . | CFH (green*) . | n . | Pearson correlation coefficient, mean ± SD (range) . | Manders overlap coefficient, mean ± SD (range) . |
|---|---|---|---|---|
| VWF | CFH | 15 | 0.359 ± 0.090 (0.261-0.543) | 0.409 ± 0.101 (0.289-0.608) |
| Fibrinogen | CFH | 24 | 0.314 ± 0.077 (0.192-0.453) | 0.381 ± 0.080 (0.262-0.524) |
| CD63 | CFH | 15 | 0.217 ± 0.038 (0.158-0.296) | 0.239 ± 0.038 (0.179-0.321) |
| LAMP-1 | CFH | 20 | 0.308 ± 0.064 (0.209-0.491) | 0.349 ± 0.067 (0.251-0.560) |
| CD61 | CFH | 32 | 0.422 ± 0.087 (0.235-0.601)† | 0.490 ± 0.081 (0.317-0.655) |
| β-Actin | CFH | 20 | 0.858 ± 0.022 (0.814-0.893)† | 0.878 ± 0.019 (0.837-0.910)‡ |
| Protein (red*) . | CFH (green*) . | n . | Pearson correlation coefficient, mean ± SD (range) . | Manders overlap coefficient, mean ± SD (range) . |
|---|---|---|---|---|
| VWF | CFH | 15 | 0.359 ± 0.090 (0.261-0.543) | 0.409 ± 0.101 (0.289-0.608) |
| Fibrinogen | CFH | 24 | 0.314 ± 0.077 (0.192-0.453) | 0.381 ± 0.080 (0.262-0.524) |
| CD63 | CFH | 15 | 0.217 ± 0.038 (0.158-0.296) | 0.239 ± 0.038 (0.179-0.321) |
| LAMP-1 | CFH | 20 | 0.308 ± 0.064 (0.209-0.491) | 0.349 ± 0.067 (0.251-0.560) |
| CD61 | CFH | 32 | 0.422 ± 0.087 (0.235-0.601)† | 0.490 ± 0.081 (0.317-0.655) |
| β-Actin | CFH | 20 | 0.858 ± 0.022 (0.814-0.893)† | 0.878 ± 0.019 (0.837-0.910)‡ |
n = number of platelets analyzed.
Significant colocalization (P < .05).
Interpreted as significant colocalization.24
Uptake of CFH by platelets in vivo
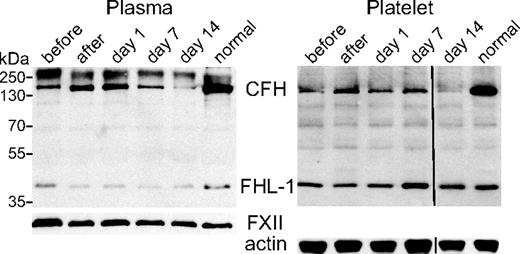
The interaction between circulating platelets and CFH in vivo was investigated by following an aHUS patient lacking constitutive CFH expression through a treatment cycle involving fresh frozen plasma transfusion (FFP; 20 mL/kg) every 14 days.21 Plasma and platelet lysates were obtained from blood samples collected immediately before and after FFP transfusion, and at posttreatment days 1, 7, and 14. Figure 5 shows the results of sequential probing of plasma and platelet lysate blots with antibody specific for CFH and FHL-1 (SCRs1-4), and either anti-FXII (plasma protein loading indicator) or anti–β-actin (platelet lysate protein loading indicator). As expected, the CFH level was low in plasma samples collected 14 days after FFP transfusion (plasma, before) rose rapidly after treatment (plasma, after), and declined between treatments (plasma, day 1, day 7, day 14). Plasma FHL-1 levels remained relatively constant throughout, consistent with endogenous synthesis. Platelet lysates (Figure 5 platelet) showed a similar pattern of variation in CFH and FHL-1 levels throughout the treatment cycle to that observed in plasma. Both platelet and plasma CFH levels appear to have remained lower in patient samples than those observed in normal control samples. The FHL-1 levels were comparable, as were levels of FXII in plasma and β-actin in platelet lysates.
Plasma and platelet CFH content in response to plasma transfusion in a constitutive CFH-null patient treated with plasma transfusion. Immunoblots (nonreduced 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis) of equivalent plasma aliquots (left) and platelet lysates (right) from a normal person (lane “normal”) and aHUS patient 1 lacking constitutive expression of CFH (but capable of expressing FHL-1) tested before therapeutic plasma transfusion (lane “before”), after treatment (lane “after”), and after treatment at days 1, 7, and 14 (corresponding lanes; transfusion interval was 14 days). Loading was equivalent to 0.7 μL of plasma and lysate from 107 platelets per lane. Immunoblots were initially probed with a rabbit polyclonal antibody capable of detecting CFH and FHL-1, and subsequently with antibodies specific for coagulation FXII for the plasma blot, and β-actin for the platelet lysate blot as loading controls. CFH is deficient in plasma and platelets before (lane “before”) and on day 14 (lane “day 14”) compared with a normal person (lane “normal”), whereas FHL-1 levels were unchanged. CFH levels were highest right after transfusion (lane “after”) and then declined over the following 14 days (lanes “day 1,” “day 7,” and “day 14”) in both plasma (left panel) and platelets (right panel). A vertical line has been inserted in the right panel (“Platelet”) to indicate a repositioned gel lane.
Plasma and platelet CFH content in response to plasma transfusion in a constitutive CFH-null patient treated with plasma transfusion. Immunoblots (nonreduced 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis) of equivalent plasma aliquots (left) and platelet lysates (right) from a normal person (lane “normal”) and aHUS patient 1 lacking constitutive expression of CFH (but capable of expressing FHL-1) tested before therapeutic plasma transfusion (lane “before”), after treatment (lane “after”), and after treatment at days 1, 7, and 14 (corresponding lanes; transfusion interval was 14 days). Loading was equivalent to 0.7 μL of plasma and lysate from 107 platelets per lane. Immunoblots were initially probed with a rabbit polyclonal antibody capable of detecting CFH and FHL-1, and subsequently with antibodies specific for coagulation FXII for the plasma blot, and β-actin for the platelet lysate blot as loading controls. CFH is deficient in plasma and platelets before (lane “before”) and on day 14 (lane “day 14”) compared with a normal person (lane “normal”), whereas FHL-1 levels were unchanged. CFH levels were highest right after transfusion (lane “after”) and then declined over the following 14 days (lanes “day 1,” “day 7,” and “day 14”) in both plasma (left panel) and platelets (right panel). A vertical line has been inserted in the right panel (“Platelet”) to indicate a repositioned gel lane.
Platelet uptake of labeled CFH in vitro
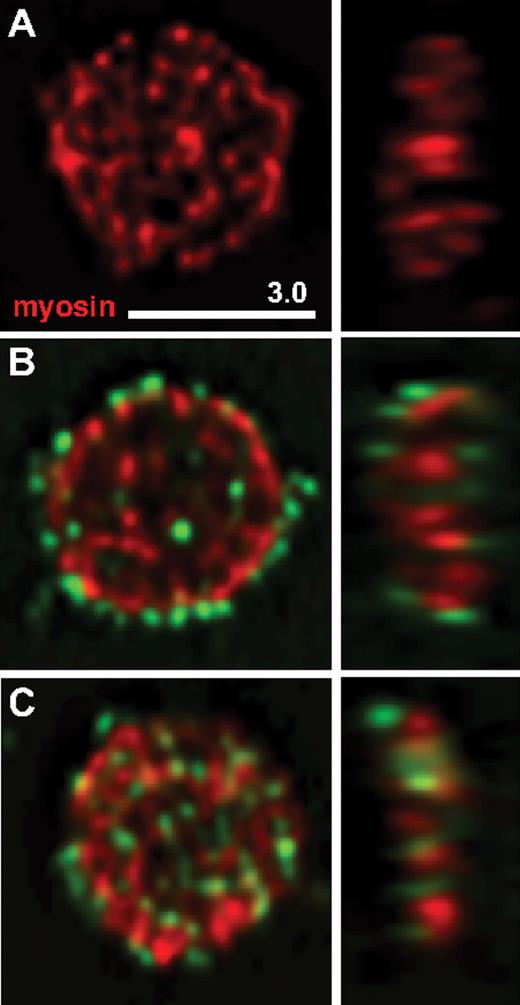
To determine whether CFH can be taken up by resting platelets in vitro, washed normal platelets were incubated with CFH covalently labeled with Alexa Fluor 488 (A488-CFH). Examination of cells after exposure to 100 μg/mL A488-CFH for up to 120 minutes revealed a pattern of early surface staining followed by increasing internalization (Figure 6). Care was taken to examine unactivated platelets, as indicated by their flat plate-like shape revealed by staining for tubulin (not shown in the figure) and myosin IIA.25 The results of the A488-CFH uptake experiments indicate that resting normal platelets with minimal levels of surface CFH (resulting from washing) rapidly take up A488-CFH on their surface, some of which is subsequently internalized.
Uptake of labeled CFH by normal washed platelets. Triple-washed platelets were incubated with or without CFH conjugated with Alexa Fluor 488 dye (A488-CFH, green color), then fixed, immunostained for platelet myosin IIA (myosin), and examined via laser fluorescence confocal microscopy. Midcell Z-slices of typical resting cells (flat, round shape with myosin staining in red) are shown in horizontal (left) and vertical (right) orientation. Platelets shown were incubated: (A) for 120 minutes in buffer; (B) with A488-CFH for 30 minutes; (C) with A488-CFH for 120 minutes. After 30 minutes, most of the platelet-associated A488-CFH was on the cell surface as indicated by peripheral staining; after 120 minutes, a substantial amount was observed inside platelets.
Uptake of labeled CFH by normal washed platelets. Triple-washed platelets were incubated with or without CFH conjugated with Alexa Fluor 488 dye (A488-CFH, green color), then fixed, immunostained for platelet myosin IIA (myosin), and examined via laser fluorescence confocal microscopy. Midcell Z-slices of typical resting cells (flat, round shape with myosin staining in red) are shown in horizontal (left) and vertical (right) orientation. Platelets shown were incubated: (A) for 120 minutes in buffer; (B) with A488-CFH for 30 minutes; (C) with A488-CFH for 120 minutes. After 30 minutes, most of the platelet-associated A488-CFH was on the cell surface as indicated by peripheral staining; after 120 minutes, a substantial amount was observed inside platelets.
Effects of aHUS patient plasma on normal platelets
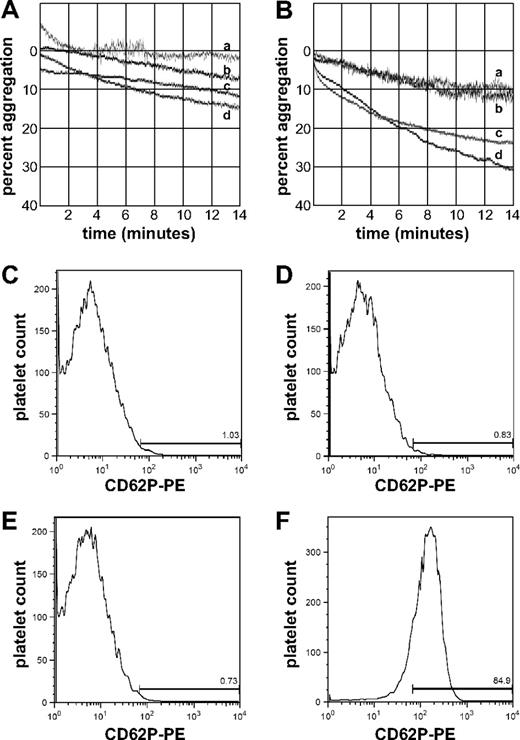
Optical platelet aggregometry with normal washed platelets treated with 50% diluted fresh autologous plasma or thawed plasma from an ABO-matched normal donor (Figure 7A) showed low levels of aggregation (7%-14% maximum aggregation; tracings c, d) after 14 minutes. Similar results were obtained (6%-11% maximum aggregation; tracings a, b) for cells preincubated with 200 μg/mL purified CFH (final concentration 100 μg/mL during aggregometry assays). When similar experiments were performed with plasma from ABO-matched aHUS patient 2 with CFH autoantibody-mediated aHUS,14 plasma obtained before FFP transfusion (Figure 7B) produced significant aggregation in the absence of added CFH (24%-29% maximum aggregation; tracings c-d), whereas CFH-treated platelets showed a baseline response (11% maximum aggregation; tracings a-b) similar to those treated with autologous or normal plasma.
Aggregation and P-selectin expression of normal platelets exposed to plasma from an aHUS patient with CFH autoantibodies. Platelet optical aggregometry tracings of normal washed platelets incubated with ABO-matched normal plasma (A) or with plasma from aHUS patient 2 (B). Platelets were either preincubated with CFH (tracings a-b) or with buffer (tracings c-d) before measuring aggregation. Flow cytometry histograms showing P-selectin expression of normal washed platelets incubated with autologous plasma (C), ABO-matched normal plasma (D), plasma from an aHUS patient (E), or autologous plasma stimulated with U46619 (F). Incubation with aHUS plasma does not induce P-selectin expression (compare C-E), whereas stimulation with U46619 does (F).
Aggregation and P-selectin expression of normal platelets exposed to plasma from an aHUS patient with CFH autoantibodies. Platelet optical aggregometry tracings of normal washed platelets incubated with ABO-matched normal plasma (A) or with plasma from aHUS patient 2 (B). Platelets were either preincubated with CFH (tracings a-b) or with buffer (tracings c-d) before measuring aggregation. Flow cytometry histograms showing P-selectin expression of normal washed platelets incubated with autologous plasma (C), ABO-matched normal plasma (D), plasma from an aHUS patient (E), or autologous plasma stimulated with U46619 (F). Incubation with aHUS plasma does not induce P-selectin expression (compare C-E), whereas stimulation with U46619 does (F).
When platelets recovered after aggregometry assays, or from parallel experiments in which cells were incubated under static conditions, were assessed for surface P-selectin expression by flow cytometry (Figure 7C-E), no significant differences were observed among treatment groups (0.73%-0.83%, compared with autologous plasma). This contrasts with platelets activated with the thromboxane analog U46619, where maximum aggregation was more than 97% (data not shown) and surface P-selectin expression was more than 84% (Figure 7F). Thus, aHUS patient plasma obtained at treatment nadir triggered an aggregation or agglutination response in normal platelets that was not associated with increased platelet P-selectin surface expression but could be eliminated by preincubation with purified CFH.
Discussion
Thrombotic microangiopathies are defined by the presence of thrombi containing fibrin and platelets in the microcirculation of various organs, and are characterized by thrombocytopenia and hemolytic anemia.15,26 Different from thrombotic thrombocytopenic purpura with cerebral involvement, in aHUS the kidney is the main target organ.27 Although aHUS has recently been linked to defects in the regulation of the alternative complement pathway, its pathogenesis is still only poorly understood.4,15 Thrombocytopenia and the presence of platelet-rich thrombi in the glomerular microvasculature of aHUS patients highlight the significance of platelets for aHUS pathology.
Platelets are the primary cellular components of the hemostatic system, and they are also intimately involved in inflammation and immune responses,28-31 wound healing and bone regeneration,32 angiogenesis,33 and a variety of pathologic conditions, most notably atherogenesis, heart attack, and stroke.29,31 In hemostasis, vascular injury stimulates platelets to adhere to several endothelial and subendothelial proteins (eg, collagen and VWF) via GP Ib/V/IX, VI, and Ia/IIa (α2β1 integrin). Secretion of platelet granule contents stimulates additional platelet recruitment, aggregation, and activation. This culminates in the exposure of the phosphatidyl serine–rich procoagulant surface to the outside of the platelet, where thrombin-dependent coagulation ensues forming a fibrin-bound platelet clot. The precise regulation of platelets to maintain a balance between a resting and an activated state is paramount to prevent both hemorrhage and inadvertent thrombosis. The prevention of inadvertent platelet activation must therefore be a strictly regulated process to minimize tissue damage. We suggest that one such regulatory mechanism involves the complement system.
Platelets, like all cells exposed to the complement system, must protect themselves from complement attack by inhibiting activation of the alternative pathway on their surface. However, there is evidence that platelet-complement interactions have some unique features. CFH, a key cell surface regulator of the constitutively active alternative complement pathway, is present in and released from platelets on activation by thrombin and/or complement.19 Terminal complement pathway components can trigger activation of platelets,34 and activated platelets have been shown capable of initiating the classic complement pathway.35,36 Activated platelets can also support the propagation of membrane attack complexes and anaphylatoxin formation on their surface, which is associated with C3b binding to P-selectin exposed on the platelet surface.17 Furthermore, CFH binds to the surface of nonactivated platelets via its C-terminal domain, either directly via the GPIIb/IIIa receptor (αIIbβ3 integrin) or indirectly via binding proteins, such as TSP1, and these protein-protein interactions are lost or impaired by C-terminal CFH mutations.20,37 Recently, serum from aHUS patients with C-terminal mutant CFH has been reported to activate platelets from normal donors.16 Thus, platelets represent a unique cellular intersection between the coagulation and complement systems in that they contain factors involved in both responses, which appear to interact in ways that are important for their independent, and possibly mutual, regulation.
It has been proposed that CFH is stored in and released from platelet α-granules,19,38 which suggests that CFH is expressed within the platelet lineage by precursor megakaryocytes and/or taken up from plasma. We began our examination of these possibilities by analyzing the protein content of megakaryocytes grown in serum-free suspension culture, normal, and ARC syndrome platelets (Figure 1). We found that, although some platelet CFH appears to originate from endogenous synthesis in megakaryocytes, α-granules are not required for its presence. Direct examination via TEM of CFH in immunogold-labeled sections from normal human platelets (Figure 2) showed CFH distributed on the cell surface and throughout the cytoplasm, with some apparent association with cell membranes but not specifically with α-granules or other recognizable vesicles.
This nongranular localization of CFH was surprising and prompted us to examine whole-cell CFH distribution in platelets via high resolution laser fluorescence spinning disk confocal microscopy. Recent advances have facilitated detailed examination of the cellular localization of platelet proteins; for example, it has been observed that megakaryocyte-synthesized VWF and plasma-derived fibrinogen are stored in distinct platelet α-granule populations.23,33 The technical challenges associated with precisely localizing proteins within platelets include their small size (0.5 μm × 3 μm), which makes it difficult to localize molecules to specific structures and organelles, and the potential for inadvertent platelet activation or other alterations during sample preparation, which can trigger changes in cell structure and the secretion of granule contents. We were able to minimize these technical challenges, as demonstrated by the ability to obtain clear images of quiescent platelets. The resting state of platelets was indicated by a flat, discoid shape (Figure 3A) with intact circumferential microtubule rings surrounding well-resolved α-granules (Figure 3B). Similar methods can be used to image platelets in various stages of activation as indicated by changes in cell shape, cytoskeletal architecture, and loss or rearrangement of granule contents (data not shown).
Our laser fluorescence spinning disk confocal microscopy studies confirmed that platelet CFH is not confined to platelet α-granule populations containing VWF or fibrinogen (Figure 3C-D; Table 1), which we observed to be distinct (data not shown) as has been shown by others.23,33 These findings are consistent with the presence of CFH in platelets devoid of α-granules (Figure 1 “ARC platelet” lane). To investigate whether CFH is localized to other platelet granules, we performed whole-platelet colocalization studies using antibodies specific for the lysosome proteins LAMP-1 and CD63, with the latter also being found in δ-granules (Figure 4A-B). Significant colocalization of CFH with δ-granules and lysosomes was not observed (Table 1). Thus, CFH is clearly present in resting platelets but is not sequestered in α-granules, δ-granules, or lysosomes.
CFH has been shown to bind to washed human platelets, and the observation that anti-GPIIb/IIIa (anti-αIIbβ3 integrin) antibodies or fibrinogen impede this interaction implicates the αIIbβ3 integrin receptor in CFH binding to the platelet surface.20 To determine whether we can detect CFH bound to resting platelet αIIbβ3 integrin receptors, we performed colocalization studies using an antibody (CD61) specific for β3 integrin. The partial colocalization of CFH with CD61 in our studies (Figure 4C; Table 1) is consistent with some CFH being bound to αIIbβ3 integrin on the surface of resting platelets.
Surprisingly, the most abundant colocalization of CFH was with β-actin (Figure 4D; Table 1). Although this is consistent with the TEM observations showing a nongranular cytoplasmic distribution within platelets (Figure 2), this was somewhat unexpected because platelet CFH has been reported to be released from agonist-stimulated platelets.19 We have also observed release of CFH from platelets stimulated with a variety of agonists (data not shown), indicating that, in addition to granule-dependent secretion, platelets may also be capable of releasing significant amounts of surface-associated and/or cytoplasmic proteins.
We demonstrated that platelets may obtain some CFH through endogenous synthesis from megakaryocytes (Figure 1 “Normal MK” lane). To investigate the interaction of platelets and CFH in vivo, we followed a CFH-null patient (patient 1) through a 14-day treatment cycle of CFH replacement therapy via plasma transfusion. A dramatic rise in plasma CFH was seen immediately after plasma transfusion followed by a steady decline (Figure 5 left panel), and platelet CFH content followed a similar pattern (Figure 5, compare left and right panels). This indicates that circulating CFH-deficient platelets were able to rapidly incorporate CFH from the plasma pool in vivo. Serving as a fortuitous internal control, the patient's plasma and platelet FHL-1 levels did not show any transfusion-influenced fluctuation, consistent with known normal expression in this patient. The similarity between the observed CFH plasma half-life of approximately 6 days21 and the average circulatory platelet life span of 7 to 10 days suggests a concentration-dependent equilibrium between these compartments.
Platelets can incorporate fibrinogen via endocytosis39 and take up other proteins, particles, viruses, and bacteria via interactions involving the cell membrane and the open canalicular system.40,41 The rapid acquisition of CFH by CFH-null platelets in vivo (within a few hours after transfusion; Figure 5 right panel, “after” lane) suggests that circulating platelets can take up plasma CFH directly (it may also be packaged into assembling platelets). Evidence for the rapid acquisition of CFH by platelets comes from our in vitro studies of normal platelets incubated with fluorescently labeled CFH (Figure 6), where CFH was localized at the platelet surface after 30 minutes (Figure 6B) and internalized after 120 minutes (Figure 6C).
Whether platelet CFH uptake occurs via endocytosis or through an active or passive uptake mechanism via the open canalicular system is unknown. The mechanism does not appear to involve platelet activation because the cells that took up labeled CFH were observed to be quiescent (Figure 6). Further studies are required to elucidate the precise mechanism of CFH uptake into platelets. Similarly, it is not clear how CFH, which is not contained in granules, is released from platelets. Work is underway to address this question, and preliminary studies indicate that agonist-stimulated platelets release CFH.
Incubation of washed resting platelets with serum from aHUS patients has been reported to cause C3 and C9 platelet deposition and P-selectin expression indicative of platelet activation in vitro.16 Attempting similar experiments, we observed that incubation of normal platelets with serum, either autologous or from ABO blood group-matched normal donors, consistently induced substantial platelet activation as assessed by optical aggregometry and surface P-selectin expression (data not shown). This observation, together with the fact that platelets normally circulate in plasma, led us to investigate the interactions between platelets and CFH using plasma from an aHUS patient (patient 2) with autoantibodies to the CFH C-terminus,14 taken before FFP transfusion (which maintains a stable clinical state with regard to platelet count and renal function). We observed a substantial increase in aggregation or agglutination of normal washed platelets exposed to aHUS plasma relative to autologous or ABO-matched normal plasma, which was abrogated by preincubation with purified CFH (Figures 7A-B). This indicates that CFH has a platelet-stabilizing role both under normal physiologic conditions and probably after FFP treatment in aHUS patients. Interestingly, P-selectin surface expression (platelet activation with α-granule release) measured by flow cytometry was not increased in platelets incubated with aHUS plasma within our observation time frame (15-30 minutes). Thus, although platelets showed a CFH-dependent in vitro aggregation or agglutination response to aHUS plasma, this response did not involve a detectable rise in α-granule secretion, indicating a distinct mode of complement-mediated platelet alteration.
We have shown that platelets can take up CFH from plasma, that the acquisition of CFH from megakaryocyte precursors may be involved in platelet loading of CFH, and that platelet CFH is distributed throughout the cytoplasmic and surface compartments rather than concentrated in vesicles. Our observations that CFH-deficient platelets rapidly take up CFH in vivo and in vitro and that restoration of CFH function (via preincubation of platelets with CFH) inhibits the ability of aHUS patient plasma to stimulate aggregation or agglutination of normal platelets in vitro indicate that platelet-CFH interactions are important for effective treatment of aHUS patients. Thus, our observations support the hypothesis that platelet-CFH interactions play a key role in aHUS pathogenesis in particular and platelet-complement interactions in general, both of which may involve aspects of platelet behavior that remain to be elucidated.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michael Kirschfink for helpful discussions.
C.L. was supported by a start-up grant from the Research Institute of The Hospital for Sick Children, Toronto, ON. W.H.A.K. was supported by an operating grant from the Canadian Institutes of Health Research (CIHR, MOP-81208) and a Phase II Clinician Scientist Award from the Heart and Stroke Foundation of Ontario (CS 5982).
The authors wish to dedicate this manuscript to Prof Dr Bernhard Roth, Children's Hospital of the University of Cologne, Cologne, Germany, on the occasion of his 60th birthday.
Authorship
Contribution: C.L. designed research, analyzed data, wrote the paper, and provided clinical care to patient 2; F.G.P. performed research and contributed to analyzing data and writing the paper; L.L. and H.C. performed research; S.H. and B.H. provided clinical care to patient 1 and contributed patient samples; D.F.G. provided clinical care to patient 2; P.F.Z. provided antibodies and contributed to writing the paper; and W.H.A.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Licht, Division of Nephrology and Program in Cell Biology, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8, Canada; e-mail.christoph.licht@sickkids.ca.