Abstract
Hematopoietic stem cell (HSC) proliferation is tightly regulated by a poorly understood complex of positive and negative cell-cycle regulatory mechanisms. Necdin (Ndn) is an evolutionally conserved multifunctional protein that has been implicated in cell-cycle regulation of neuronal cells. Here, we provide evidence that necdin plays an important role in restricting excessive HSC proliferation during hematopoietic regeneration. We identify Ndn as being preferentially expressed in the HSC population on the basis of gene expression profiling and demonstrate that mice deficient in Ndn show accelerated recovery of the hematopoietic system after myelosuppressive injury, whereas no overt abnormality is seen in steady-state hematopoiesis. In parallel, after myelosuppression, Ndn-deficient mice exhibit an enhanced number of proliferating HSCs. Based on these findings, we propose that necdin functions in a negative feedback loop that prevents excessive proliferation of HSCs during hematopoietic regeneration. These data suggest that the inhibition of necdin after clinical myelosuppressive treatment (eg, chemotherapy, HSC transplantation) may provide therapeutic benefits by accelerating hematologic recovery.
Introduction
Hematopoietic stem cells (HSCs) play a central role in the regulation of hematopoietic homeostasis.1 HSCs use their self-renewal and multipotential differentiation abilities to sustain a lifelong blood supply and to replenish the hematopoietic system when injured.2 Decades of intensive study of HSCs have allowed investigators to identify these cells as being present within a small population of CD34lo/− Kit+ Sca1+ lineage-marker-negative (CD34−/low KSL) cells in the mouse bone marrow (BM), and thereby enabled the prospective isolation of nearly homogeneous HSC populations for further characterization.3 Transplantation of CD34−/low KSL cells has demonstrated that a single HSC is sufficient to provide long-term reconstitution of the entire hematopoietic system in multiple recipients, proving the remarkable regeneration capacity of HSCs.3,4 This ability holds many promises for clinical applications of stem cell replacement therapies in treatment of various hematologic disorders, such as leukemias and autoimmune diseases.
Under steady-state conditions, the majority of HSCs are maintained in a quiescent state, where they infrequently divide to produce proliferative progenitors, which eventually give rise to mature hematopoietic cells that sustain blood homeostasis.5 In contrast, in response to myelosuppressive stresses, such as myeloablative chemotherapy or irradiation, HSCs are capable of undergoing intensive proliferation to produce massive numbers of primitive progenitor cells, thereby enabling rapid hematologic regeneration.6 However, once recovery from myelosuppression has been achieved, the activated HSCs eventually return to quiescence through various negative feedback mechanisms.7 Accumulating evidence indicates that the proliferation of HSCs is strictly regulated by a complex of positive and negative mechanisms both in cell-autonomous and non–cell-autonomous fashions.8 For instance, several cytokines and growth factors, such as stem cell factor, thrombopoietin, and Wnt, have been implicated in promoting HSC cell-cycle progression, whereas HSC cell cycling is negatively regulated by several intrinsic factors, including Foxo3a, Gfi1, Mef, p21Cip, suppressor of cytokine signaling, and Sprouty/Spred.9-15 Recent studies have postulated the importance of negative cell-cycle regulators in the proper maintenance of HSCs.16 Defects in such negative regulation can result in hyperproliferation of stem/progenitor cells, leading to exhaustion of the HSC pool as well as increased incidence of malignant transformation.
Understanding the molecular mechanisms underlying HSC regulation is of great importance to both basic biology and the development of clinical applications of HSCs. One approach to identify the components of the molecular pathways underlying HSC regulation is to define the molecular signature of the HSC by performing comparative transcriptional profiling of distinct subsets of hematopoietic cells. In the past decade, several attempts have been made by independent investigators to define the molecular signature of HSCs.7,17-22 Although accumulation of such information has more or less uncovered the molecular makeup of HSCs, it is still laborious to elucidate the physiologic functions of each individual gene in the regulation of HSCs.
Necdin (Ndn) is a member of melanoma antigen family of molecules, whose physiologic roles have not been well characterized.23 Necdin has been implicated in cell-cycle control and apoptosis in neuronal cells.24,25 Intriguingly, recent genetic analyses have revealed that aberrant genomic imprinting of NDN on the human 15q11-q13 chromosomal region is, at least in part, responsible for the pathogenesis of Prader-Willi syndrome, but it remains obscure how necdin ablation results in Prader-Willi syndrome.26-28 Interestingly, the involvement of necdin in the regulation of HSCs has been suggested previously, although its physiologic role has yet to be explained.29
Here we have performed a stringent comparative gene profiling analysis to identify genes preferentially expressed in the HSC population. This led us to focus on 3 putative cell-cycle regulators, including Necdin, Klf9, and Nupr1.30-32 We analyzed the phenotype of the mice rendered null for these genes and found that mice lacking Ndn show abnormal hematologic recovery after myelosuppressive treatment. Although our results are partly consistent with the previous report showing the role of necdin in the maintenance of HSC quiescence and self-renewal,29 our in vivo data indicate that the negative cell-cycle regulatory function of necdin is largely restricted to the regeneration phase of hematopoiesis. Hence, our findings suggest a novel therapeutic opportunity: the inhibition of necdin function to enhance hematologic recovery after myeloablative chemotherapy or HSC transplantation.
Methods
Mice
C57BL/6 (B6-CD45.2) mice were purchased from Japan SLC Inc. C57BL/6-CD45.1 (purchased from Sankyo-Lab Service), Nupr1(p8)-deficient mice32 (gift from Dr J. Iovanna, Marseille, France), Klf9 (Bteb1)-deficient mice33 (gift from Dr Y. Fujii-Kuriyama, Tsukuba, Japan), and C57BL/6-CD45.1;CD45.2 F1 mice were bred and maintained in the RIKEN CDB facility. Ndn-deficient mice (Ndntm1Ky, ICR background) were generated and maintained as described previously.34 All these mutant mice were backcrossed with C57BL/6N mice at least 5 generations. Given that Ndn is a maternally imprinted gene, we crossed heterozygous male mice (Ndn+/−) with wild-type C57BL/6 female mice (B6-CD45.2) to obtain wild-type (Ndn+/+) and paternal Ndn-deficient (Ndn+m/−p) mice. Loss of Ndn expression in HSCs of Ndn+m/−p mice was confirmed by quantitative reverse-transcription polymerase chain reaction (PCR; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). All mice used were produced by crossing heterozygous males with wild-type females (ensuring the paternal origin of the mutant allele), and heterozygotes were identified by genomic PCR using primers listed in supplemental Table 1. All animal experiments were performed in accordance with the guidelines of the RIKEN Center for Developmental Biology for animal and recombinant DNA experiments and with the approval of the RIKEN Center for Developmental Biology institutional review board.
Blood count
Peripheral blood was collected and analyzed on an automated blood cell counter, KX-21 (Sysmex), according to the manufacturer's instructions.
Purification of mouse CD34−/low KSL and CD34+ KSL cells
CD34−/low KSL and CD34+ KSL cells were isolated as described previously.3 In brief, low-density cells were isolated on Histopaque 1.083 (Sigma-Aldrich). The cells were stained with an antibody cocktail consisting of biotinilated anti–Mac-1, –Gr-1, –Ter119, –B220, –CD4, and –CD8 monoclonal antibodies (BD Biosciences PharMingen). Lineage-positive cells were depleted with Streptavidin Particles Plus-DM (BD Biosciences PharMingen). The remaining cells were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD34 (BD Biosciences PharMingen), phycoerythrin (PE)–conjugated anti–Sca-1 (BD Biosciences PharMingen), and allophycocyanin (APC)–conjugated anti–c-Kit (clone ACK235 or 2B8; BD Biosciences PharMingen) antibodies. Biotinylated antibodies were detected with streptavidin-PE Cy7 (BD Biosciences PharMingen). The stained cells were analyzed and isolated on a FACSAria (BD Biosciences).
Microarray analysis
RNA was harvested from 5 × 104 CD34−/low KSL cells or 1 × 105 CD34+ KSL cells, using the RNeasy Micro kit (QIAGEN) according to the manufacturer's procedure. Total RNA from each population was subjected to one round of linear amplification using T7-based in vitro transcription (MessageAmp II aRNA Amplification kit, Ambion). cRNA probe preparation and hybridization to Affymetrix oligonucleotide arrays MGU74v2 A, B, and C were performed as described previously.36 The expression data were analyzed using the eXintegrator system (http://www.cdb.riken.jp/scb/documentation/) and dChip software (http://biosun1.harvard.edu/complab/dchip/). Probe sets were first sorted on the basis of the ratio of expression in CD34−/low to CD34+ fraction using the dChip expression estimate. The expression of identified genes was then examined in an extended dataset (containing a range of blood types), using the eXintegrator system, to select genes specifically expressed in the stem cell compartment. Raw data are available for download from Array Express (http://www.ebi.ac.uk/microarray-as/aer/login, accession number: E-MEXP-2223).
Quantitative PCR
Total RNA was harvested from FACS-purified CD34−/low KSL cells, CD34+ KSL progenitor cells, Gr-1+ neutrophils, Mac-1+ monocytes/macrophages, B220+ B cells, thymic CD4+ CD8+ T cells, TER119+ erythroblasts, or CD41+ megakaryocytes. Total RNA was then subjected to one round of in vitro transcription as described in the previous paragraph. Quantitative PCR was performed using a QuantiTect SYBR Green PCR kit (QIAGEN) according to the manufacturer's protocol. Each sample was normalized to hypoxanthine phosphoribosyl transferase. Primer sequences are described in supplemental Table 1.
Immunocytochemistry
CD34−/low KSL and CD34+ KSL cells were sorted and fixed with Methacarn fixation solution, followed by centrifugation onto glass slides using Cytospin (Shandon). The cells were stained with the following antibodies: rabbit anti-necdin (NC243)37 and anti–mouse β-actin antibodies. Staining was performed using specific secondary antibodies conjugated to Alexa 488 or 546. TOPRO-3 (Invitrogen) was used to detect nuclei. Fluorescent images were obtained using a confocal laser scanning microscope (Carl Zeiss LSM 510 system).
Hematopoietic progenitor assays in vitro and in vivo
Colony-forming activity (CFU-C) assays were performed using MethoCult M3434 (Stem Cell Technologies). A total of 2 × 104 BM mononuclear cells (MNCs) were plated on 35-mm culture dishes and then incubated at 37°C in humidified chambers containing 5% CO2. Colonies were counted using a dissecting microscope after 10 to 14 days of culture. For CFU-S12 assay, 1 × 105 BM MNCs were injected intravenously into lethally irradiated mice. The spleens were removed on day 12 after injection and fixed in Bouin's solution. Colonies were counted under a dissecting microscope.
Long-term competitive reconstitution assay
Test cells (either 4 × 105 BM MNCs, 1 × 103 KSL cells, or 20 CD34−/low KSL cells) from B6-CD45.2 mice were mixed with 4 × 105 CD45.1/CD45.2 adult BM MNCs and injected into adult recipient mice (B6-CD45.1) irradiated at a dose of 9.5 Gy using a Cesium137 GammaCell40 Exactor Irradiator (MDS Nordia). Peripheral blood cells were stained with FITC-conjugated anti-CD45.1 antibodies, biotinylated anti-CD45.2 antibodies followed by APC-Cy7 streptavidin, PE-conjugated anti Mac-1 and Gr-1 antibodies, APC-conjugated anti-CD4 and -CD8 antibodies, and PE-Cy7–conjugated anti-B220 antibodies. The test cell-derived chimerism was evaluated on a FACSCanto (BD Bioscience) system.
Cell-cycle analysis
Pyronin Y staining was performed as described previously.38 BM cells were resuspended in ice-cold staining medium and stained for 30 minutes on ice with the following monoclonal antibodies: biotinylated lineage markers (Gr-1, Mac-1, B220, CD4, CD8, TER119), Pacific Blue-conjugated CD34 (eBioscience), PE-Cy7-conjugated Sca-1 (eBioscience), and APC-conjugated c-Kit. Biotinylated antibodies were visualized with APC-Cy7 streptavidin. Cell-cycle status was analyzed using a FACSAria.
BrdU incorporation assay
Mice were fed water containing 1 mg/mL of BrdU (nakalai tesque) for 7 days. BM cells were isolated and lineage-negative selection was done using the BD IMag kit (BD Biosciences PharMingen) and then stained with CD34, Sca-1, c-Kit antibodies. Proliferating cells were identified by BrdU incorporation assay using the FITC BrdU flow kit (BD Biosciences PharMingen).
Apoptosis assay
Apoptosis assays were done by staining cells with 7-amino-actinomycin D (7-AAD) and annexin V (BD Biosciences PharMingen) in 3 different experimental settings. First, CD34−/low KSL cells were cultured in serum-supplemented medium in the absence of cytokines and apoptosis was measured after 0, 24, or 48 hours of culture. The second settings is apoptosis induced, by ionizing irradiation and the third is cyclophosphamide-induced apoptosis.29,39 Apoptosis of the HSC population was analyzed 12 hours after whole-body irradiation (6.5 Gy) or 2 days after intraperitoneal injection of cyclophosphamide (200 mg/kg).
Administration of 5-FU
5-Fluorouracil (5-FU; Sigma-Aldrich) was administered to mice intravenously at a dose of 200 mg/kg. To follow hematopoietic recovery, peripheral blood counts were monitored at 3-day intervals. For weekly 5-FU treatment, 5-FU was injected intraperitoneally at a dose of 150 mg/kg weekly and survival was monitored daily.
Results
Identification of HSC-specific nuclear factors
In this study, we used a global gene expression profiling approach to identify genes that are preferentially expressed in the HSC population. It has been demonstrated that the most primitive HSCs are predominantly present in the CD34−/low c-Kit+ Sca-1+ lineage-marker-negative (hereafter KSL) population but are undetectable in other subpopulations of BM cells.3 Hence, we hypothesized that genes involved in the regulation of HSCs would be preferentially expressed in the CD34−/low KSL population. To identify such HSC-specific genes, a stringent comparative gene expression profiling was carried out by combining microarray analysis and subsequent quantitative PCR screens across a range of hematopoietic cell lineages, including CD34+ KSL progenitor cells, Gr-1+ neutrophils, Mac-1+ monocytes/macrophages, B220+ B cells, thymic CD4+ CD8+ T cells, TER119+ erythroblasts, and CD41+ megakaryocytes (supplemental Table 2). This analysis allowed us to identify a total of 32 genes whose transcripts were at least 2 times more highly expressed in the CD34−/low KSL HSCs than in 9 distinct subsets of differentiated hematopoietic lineages (supplemental Table 2). Notably, the gene list described here shows remarkable overlap with previously published literature (20 of the 32 genes, including Serpina3g, Cldn5, Jam2, Vcam1, Cdkn1c, Fzd4, Iigp1, Mpdz, Nrk, Socs2, Cyp4b1, Fabp4, Itsn, Bmp2, Tfpi, Timp3, Foxa3, Klf9, Mllt3, and Ndn), supporting the validity of our gene expression analysis.7,17-22
This gene list contains 6 nuclear factors, including Foxa3, Klf9, Mllt3, Ndn, Nupr1, and Rxrg (supplemental Figure 2). It has been demonstrated that nuclear factors play predominant roles in the regulation of HSCs.8 Hence, we focused on these nuclear factors to further characterize their physiologic functions in HSCs. Of these 6 nuclear factors, Klf9, Ndn, and Nupr1 became the focus of our further studies because of their potential implications in cell-cycle control.30-32 We obtained null mutant mice for these 3 genes and investigated the effects of the gene ablation on hematopoiesis. These mutants exhibited no overt abnormalities during steady-state hematopoiesis; BM cellularity and the frequencies of CFU-C and CFU-S12 in these mutants were indistinguishable from wild-type (supplemental Tables 3, 4). In contrast, competitive repopulation assays revealed that the Ndn-deficient BM cells showed an accelerated short-term hematopoietic reconstitution, whereas the repopulation capacity of Klf9-deficient or Nupr1-deficient BM cells were comparable with that of the wild-type cells (supplemental Figure 3). Thus, we concluded that, despite the preferential expression of Klf9 and Nupr1 in HSCs, Klf9 and Nupr1 are dispensable for the regulation of HSCs. Based on these data, we focused on necdin for further functional characterization.
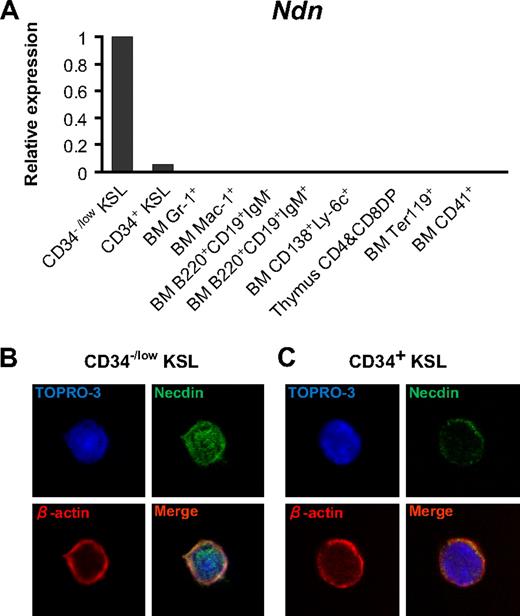
Immunocytochemical staining showed that necdin protein was present both in the nucleus and cytoplasm of freshly isolated CD34−/low KSL cells (Figure 1B). On the other hand, whereas overall expression was low (Figure 1A), necdin immunoreactivity was detected only in the cytoplasm of CD34+ KSL cells (Figure 1C). Although we have no data to indicate a function for this relocation, this observation is consistent with recent reports describing shuttling of necdin in postmitotic neurons.40
Preferential expression of necdin in HSCs. (A) Ndn expression in distinct subsets of hematopoietic cells. Ndn expression was quantified by quantitative PCR assay in each subset of hematopoietic cells as indicated in the graph. Levels of Ndn expression were normalized to an internal control (Hprt) gene. Expression of Ndn is shown calculated relative to Ndn expression in CD34−/low KSL cells. (B-C) Localization of necdin in HSCs. Freshly isolated CD34−/low KSL cells (B) or CD34+ KSL cells (C) were stained with anti-necdin (green), anti–β-actin (red), and TO-PRO3 (nuclei, blue).
Preferential expression of necdin in HSCs. (A) Ndn expression in distinct subsets of hematopoietic cells. Ndn expression was quantified by quantitative PCR assay in each subset of hematopoietic cells as indicated in the graph. Levels of Ndn expression were normalized to an internal control (Hprt) gene. Expression of Ndn is shown calculated relative to Ndn expression in CD34−/low KSL cells. (B-C) Localization of necdin in HSCs. Freshly isolated CD34−/low KSL cells (B) or CD34+ KSL cells (C) were stained with anti-necdin (green), anti–β-actin (red), and TO-PRO3 (nuclei, blue).
Because Ndn is a maternally imprinted gene,26 necdin expression in somatic cells should be exclusively derived from the paternal allele. Although this imprinting should occur in all somatic cells, it has not been demonstrated in HSCs. We therefore confirmed the maternal inactivation of the Ndn allele. For this, Ndn expression in HSCs was compared between wild-type mice and heterozygous mutant mice carrying a Ndn-null allele on the paternal chromosome (Ndn+m/−p). In contrast to wild-type HSCs where intensive Ndn expression was detectable, its expression was virtually undetectable in Ndn+m/−p HSCs (supplemental Figure 1), indicating inactivation of maternal-derived Ndn. These data confirm the lack of expression of the Ndn gene in Ndn+m/−p HSCs. Hence, we used Ndn+m/−p mice as Ndn-deficient mice.
Necdin deficiency does not alter steady-state hematopoiesis
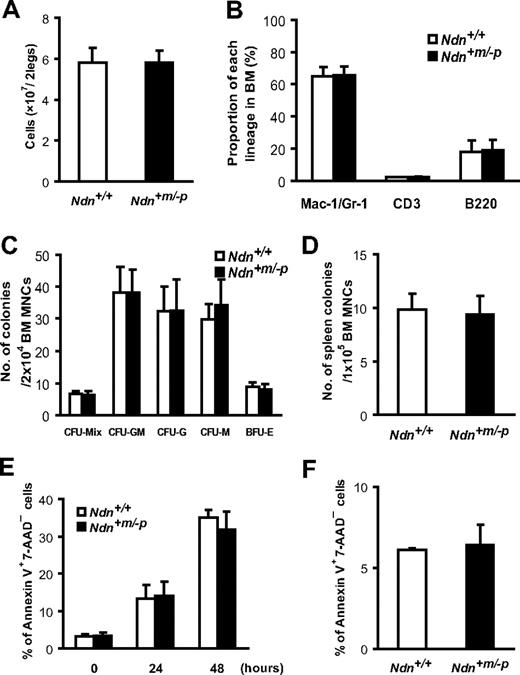
To examine the effects of necdin deficiency on steady-state hematopoiesis, we analyzed the BM and peripheral blood of 10- to 12-week-old Ndn+m/−p mice. Ndn+m/−p mice exhibited normal BM cellularity and peripheral blood cell counts (Figure 2A; Table 1). Flow cytometric analyses indicated that the frequencies of myeloid cell (Mac-1/Gr-1+), B-cell (B220+), and T-cell (CD3+) populations in Ndn+m/−p BM were similar to those in wild-type BM (Figure 2B). To assess the differentiation and proliferation capacities of Ndn-deficient hematopoietic progenitor cells, both in vitro and in vivo colony-forming assays were performed using BM MNCs obtained from either Ndn+/+ or Ndn+m/−p mice. The in vitro CFU-C of Ndn+m/−p BM MNCs were equivalent to those of Ndn+/+ cells (Figure 2C). The capacity of Ndn+m/−p BM MNCs to form colonies in the spleen 12 days after transplantation (CFU-S12) was also comparable with that of wild-type BM MNCs (Figure 2D). Although we cannot exclude the possibility that different experimental conditions would reveal major differences between wild-type and Ndn-deficient cells, these data suggest that necdin is dispensable for the differentiation and proliferation potential of hematopoietic progenitor cells during steady-state conditions.
Necdin is dispensable for steady-state hematopoiesis. (A) Normal BM cellularity in Ndn+m/−p mice. BM cells were isolated from the femurs and tibias of both hind legs. Absolute numbers of BM cells obtained from either Ndn+/+ or Ndn+m/−p mice were determined with a hemocytometer. Data shown are the mean number ± SD (n = 11). (B) Ndn-deficient mice exhibit normal myeloid, T-cell, and B-cell differentiation capacities. BM cells isolated from either Ndn+/+ or Ndn+m/−p mice were stained with anti-Mac1 and Gr-1 antibodies, anti-CD3 antibody, or anti-B220 antibody. The frequency of myeloid cells (Mac-1+/Gr-1+), T cells (CD3+), or B cells (B220+) in BM was determined by flow cytometry. Data shown are the mean number ± SD (n = 4). (C) Normal in vitro colony-forming capacity of Ndn+m/−p BM cells. BM cells from either Ndn+/+ or Ndn+m/−p mice were assessed by an in vitro colony formation assay (“Hematopoietic progenitor assays in vitro and in vivo”). Data are shown as the mean number ± SD of colonies (n = 4). (D) Colony-forming units in spleen at day 12 after transplantation (CFU-S12) are comparable between Ndn+/+ and Ndn+m/−p BM cells. Data are shown as mean colony number ± SD. No significant difference was observed between Ndn+/+ and Ndn+m/−p mice (Ndn+/+: n = 8, Ndn+m/−p: n = 5). (E) The frequency of apoptotic cells in CD34−/low KSL cells after cytokine depletion. FACS-purified CD34−/low KSL cells from Ndn+/+ and Ndn+m/−p mice were cultured without cytokines in serum-containing medium for the indicated times and stained with annexin V and 7-AAD. Data shown are the mean percentage ± SD of annexin V+ 7-AAD− cells (n = 3). No significant differences were detected between Ndn+/+ and Ndn+m/−p mice. (F) Apoptosis rates in BM cells after γ-irradiation. BM cells from Ndn+/+ or Ndn+m/−p mice harvested 12 hours after 6.5 Gy irradiation were evaluated for apoptosis using annexin V staining. Data are shown as the mean percentage ± SD of annexin V+ 7-AAD− CD34−/low KSL cells (n = 3). No significant differences were seen between Ndn+/+ and Ndn+m/−p mice.
Necdin is dispensable for steady-state hematopoiesis. (A) Normal BM cellularity in Ndn+m/−p mice. BM cells were isolated from the femurs and tibias of both hind legs. Absolute numbers of BM cells obtained from either Ndn+/+ or Ndn+m/−p mice were determined with a hemocytometer. Data shown are the mean number ± SD (n = 11). (B) Ndn-deficient mice exhibit normal myeloid, T-cell, and B-cell differentiation capacities. BM cells isolated from either Ndn+/+ or Ndn+m/−p mice were stained with anti-Mac1 and Gr-1 antibodies, anti-CD3 antibody, or anti-B220 antibody. The frequency of myeloid cells (Mac-1+/Gr-1+), T cells (CD3+), or B cells (B220+) in BM was determined by flow cytometry. Data shown are the mean number ± SD (n = 4). (C) Normal in vitro colony-forming capacity of Ndn+m/−p BM cells. BM cells from either Ndn+/+ or Ndn+m/−p mice were assessed by an in vitro colony formation assay (“Hematopoietic progenitor assays in vitro and in vivo”). Data are shown as the mean number ± SD of colonies (n = 4). (D) Colony-forming units in spleen at day 12 after transplantation (CFU-S12) are comparable between Ndn+/+ and Ndn+m/−p BM cells. Data are shown as mean colony number ± SD. No significant difference was observed between Ndn+/+ and Ndn+m/−p mice (Ndn+/+: n = 8, Ndn+m/−p: n = 5). (E) The frequency of apoptotic cells in CD34−/low KSL cells after cytokine depletion. FACS-purified CD34−/low KSL cells from Ndn+/+ and Ndn+m/−p mice were cultured without cytokines in serum-containing medium for the indicated times and stained with annexin V and 7-AAD. Data shown are the mean percentage ± SD of annexin V+ 7-AAD− cells (n = 3). No significant differences were detected between Ndn+/+ and Ndn+m/−p mice. (F) Apoptosis rates in BM cells after γ-irradiation. BM cells from Ndn+/+ or Ndn+m/−p mice harvested 12 hours after 6.5 Gy irradiation were evaluated for apoptosis using annexin V staining. Data are shown as the mean percentage ± SD of annexin V+ 7-AAD− CD34−/low KSL cells (n = 3). No significant differences were seen between Ndn+/+ and Ndn+m/−p mice.
Peripheral blood cell count in Ndn+/+ and Ndn+m/−p mice
| . | Ndn+/+ . | Ndn+m/−p . | P . |
|---|---|---|---|
| White blood cells, per μL | 6247 ± 1684 | 7222 ± 1841 | .11 |
| Red blood cells, ×104/μL | 912 ± 71.4 | 944 ± 37.7 | .11 |
| Hemoglobin, g/dL | 13.4 ± 1.1 | 14.0 ± 0.5 | .09 |
| Hematocrit, percentage | 46.3 ± 4.3 | 48.0 ± 2.3 | .15 |
| MCV, fL | 50.7 ± 1.4 | 50.9 ± 1.3 | .70 |
| MCH, pg | 14.7 ± 0.4 | 14.8 ± 0.5 | .86 |
| MCHC, g/dL | 29.0 ± 1.2 | 29.1 ± 1.2 | .86 |
| Platelets, ×104/μL | 105.4 ± 22.0 | 102.0 ± 17.0 | .61 |
| . | Ndn+/+ . | Ndn+m/−p . | P . |
|---|---|---|---|
| White blood cells, per μL | 6247 ± 1684 | 7222 ± 1841 | .11 |
| Red blood cells, ×104/μL | 912 ± 71.4 | 944 ± 37.7 | .11 |
| Hemoglobin, g/dL | 13.4 ± 1.1 | 14.0 ± 0.5 | .09 |
| Hematocrit, percentage | 46.3 ± 4.3 | 48.0 ± 2.3 | .15 |
| MCV, fL | 50.7 ± 1.4 | 50.9 ± 1.3 | .70 |
| MCH, pg | 14.7 ± 0.4 | 14.8 ± 0.5 | .86 |
| MCHC, g/dL | 29.0 ± 1.2 | 29.1 ± 1.2 | .86 |
| Platelets, ×104/μL | 105.4 ± 22.0 | 102.0 ± 17.0 | .61 |
Data from CBC counts of peripheral blood collected by retro-orbital bleeding of Ndn+/+ and Ndn+m/−p mice. Values shown are mean ± SD (n = 18).
In neurons, it has been reported that necdin plays a protective role against apoptosis.41 We therefore asked whether necdin might be also involved in the regulation of HSC apoptosis. To address this question, the frequencies of apoptotic cells in the Ndn+m/−p HSC population were assessed by annexin V antibody staining under various experimental settings. First, we evaluated the apoptosis of CD34−/low KSL cells in vitro. In the absence of cytokines, CD34−/low KSL cells undergo apoptosis, but the kinetics were not different between Ndn+/+ and Ndn+m/−p CD34−/low KSL cells (Figure 2E). It is known that HSCs undergo extensive apoptosis after γ-ray irradiation or treatment with genotoxic drugs. To examine the role of necdin during radiation-induced apoptosis of HSCs, Ndn+/+ and Ndn+m/−p mice were irradiated at a dose of 6.5 Gy, and apoptotic cells were assessed in the CD34−/low KSL population 12 hours after irradiation. As shown in Figure 2F, no significant differences in apoptotic frequencies were detectable between Ndn+/+ and Ndn+m/−p cells. Likewise, we could not detect a significant difference in the apoptosis induced by cyclophosphamide between wild-type and Ndn-deficient mice (supplemental Figure 4). Thus, these data indicate that, despite the antiapoptotic function of necdin in the neuronal lineage, the role of necdin in the regulation of HSC apoptosis appears to be rather negligible.
Ndn-deficient HSCs exhibit a normal cell-cycle status
It has been reported that necdin plays a role in promoting cell-cycle exit in postmitotic neurons.31 As HSCs are maintained in the G0 stage of the cell cycle under homeostatic conditions,5 we hypothesized that necdin might play a negative role in HSC cell-cycle regulation.
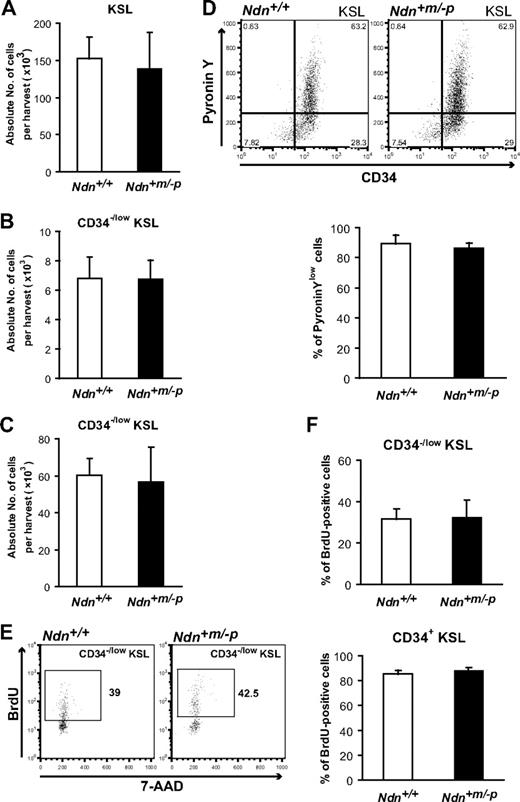
It has been shown that aberrant HSC cell-cycle regulation results in abnormal HSC and progenitor cell population sizes.16 We therefore compared the absolute numbers of HSCs and progenitor cells in Ndn+/+ and Ndn+m/−p BM. Flow cytometric analyses demonstrate that the absolute numbers of CD34−/low KSL HSCs and KSL progenitor cells in Ndn+m/−p BM cells were similar to those of Ndn+/+ BM cells (Figure 3A-B).
Necdin deficiency does not affect cell-cycle status of HSCs under homeostatic conditions. (A) Normal numbers of KSL cells in the BM of young Ndn+m/−p mice. c-Kit+ Sca-1+ cells in the Lin− fraction (KSL cells) were detected by flow cytometry. Data are shown as mean absolute number ± SD (n = 11). (B-C) Normal numbers of CD34−/low KSL in the BM of young and old Ndn+m/−p mice. c-Kit+ Sca-1+ cells in the Lin− CD34−/low fraction (CD34−/low KSL cells) of young (10-12 weeks) and old (18 months) mice were determined by flow cytometry. Data are shown as mean absolute number ± SD (young: n = 11, old: n = 3). (D) Normal frequency of quiescent CD34−/low KSL cells in Ndn+m/−p mice. BM cells from Ndn+/+ and Ndn+m/−p mice were stained with Pyronin Y, anti-CD34, c-Kit, Sca-1, and lineage markers. (Top panels) Representative pattern of 5 independent experiments. (Bottom panel) Data are shown as mean percentages ± SD of Pyronin Ylow CD34−/low KSL cells (n = 5). (E-F) Normal BrdU incorporation in Ndn+m/−p HSCs. BrdU (1 mg/mL) was administered orally to Ndn+/+ and Ndn+m/−p mice for 7 days, and BrdU incorporation in CD34−/low and CD34+ KSL cells was evaluated using anti-BrdU antibodies. (E) Representative pattern of BrdU+ CD34−/low KSL cells. (F) Data shown are the mean percentages ± SD of BrdU+ cells in the CD34−/low KSL (top panel) or CD34+ KSL cells (bottom panel). Ndn+/+: n = 5; Ndn+m/−p: n = 4.
Necdin deficiency does not affect cell-cycle status of HSCs under homeostatic conditions. (A) Normal numbers of KSL cells in the BM of young Ndn+m/−p mice. c-Kit+ Sca-1+ cells in the Lin− fraction (KSL cells) were detected by flow cytometry. Data are shown as mean absolute number ± SD (n = 11). (B-C) Normal numbers of CD34−/low KSL in the BM of young and old Ndn+m/−p mice. c-Kit+ Sca-1+ cells in the Lin− CD34−/low fraction (CD34−/low KSL cells) of young (10-12 weeks) and old (18 months) mice were determined by flow cytometry. Data are shown as mean absolute number ± SD (young: n = 11, old: n = 3). (D) Normal frequency of quiescent CD34−/low KSL cells in Ndn+m/−p mice. BM cells from Ndn+/+ and Ndn+m/−p mice were stained with Pyronin Y, anti-CD34, c-Kit, Sca-1, and lineage markers. (Top panels) Representative pattern of 5 independent experiments. (Bottom panel) Data are shown as mean percentages ± SD of Pyronin Ylow CD34−/low KSL cells (n = 5). (E-F) Normal BrdU incorporation in Ndn+m/−p HSCs. BrdU (1 mg/mL) was administered orally to Ndn+/+ and Ndn+m/−p mice for 7 days, and BrdU incorporation in CD34−/low and CD34+ KSL cells was evaluated using anti-BrdU antibodies. (E) Representative pattern of BrdU+ CD34−/low KSL cells. (F) Data shown are the mean percentages ± SD of BrdU+ cells in the CD34−/low KSL (top panel) or CD34+ KSL cells (bottom panel). Ndn+/+: n = 5; Ndn+m/−p: n = 4.
It has been demonstrated that the number of CD34−/low KSL HSCs gradually increases during aging.42 Hence, we next asked whether loss of Ndn might affect the age-associated accumulation of HSCs. We found no significant difference in the absolute number of CD34−/low KSL HSCs between Ndn+/+ and Ndn+m/−p BM of aged mice (Figure 3C). These data indicate that necdin deficiency does not affect the HSC pool size in young or old mice.
To directly assess the cell-cycle status of Ndn+m/−p HSCs, the frequency of G0 cells in the CD34−/low KSL population was determined by Pyronin Y staining. CD34−/low KSL cells from either Ndn+/+ or Ndn+m/−p mice were stained with Pyronin Y, and the frequency of G0 cells was measured by flow cytometry. Consistent with previous reports,9 we found that most of Ndn+/+ CD34−/low KSL cells (89.2% ± 6.1%) were present within the Pyronin Ylow fraction. This frequency was almost identical in Ndn+m/−p mice (86.3% ± 3.4%; Figure 3D). Next, we performed a BrdU incorporation assay to assess the frequency of actively proliferating cells in the HSC and progenitor populations. In agreement with the Pyronin Y assay, no significant differences were observed between Ndn+/+ and Ndn+m/−p mice in the frequencies of BrdU+ CD34−/low KSL cells and CD34+ KSL cells (Figure 3E-F).
Taken together, these data indicate that, despite the preferential expression of necdin in HSCs, necdin appears to be dispensable for the homeostatic regulation of HSCs in steady-state hematopoiesis.
Enhanced reconstitution ability of Ndn-deficient HSCs during hematopoietic recovery phase
Our data suggest that necdin has a limited, if any, function in steady-state hematopoiesis. Next, we asked whether necdin plays a role during hematopoietic regeneration after myelotoxic injury. Given the negative cell-cycle regulatory role of necdin in neuronal cells,30 we considered that necdin might play a role during myelosuppressive conditions, perhaps serving as a negative regulator of HSC proliferation during hematopoietic regeneration. Defects in such negative feedback mechanisms could result in abnormally rapid proliferation of hematopoietic progenitors, leading to enhanced hematologic recovery.
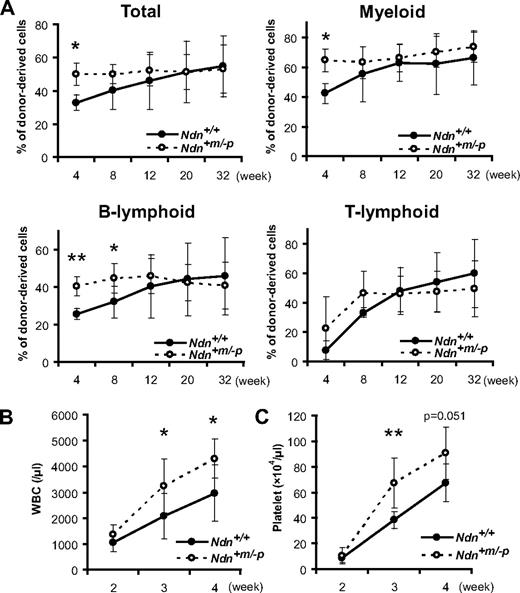
To test this possibility, the hematopoietic reconstitution capacity of Ndn+m/−p HSCs was evaluated using competitive repopulation assays. For this, 1 × 103 KSL cells isolated from either Ndn+m/−p or Ndn+/+ BM (CD45.2) were transplanted into lethally irradiated recipient animals (CD45.1) along with 4 × 105 CD45.1;CD45.2 BM MNCs. At 4 weeks after transplantation, mice transplanted with Ndn+m/−p KSL cells showed significantly higher hematopoietic reconstitution in both the myeloid and B lymphocyte populations than those that received Ndn+/+ KSL cells. However, this enhancement in hematopoietic recovery was transient, and no differences were found between mutant and wild-type mice 20 weeks after transplantation (Figure 4A). Similarly, this transient enhancement of hematologic recovery was also evident when 20 Ndn+m/−p CD34−/low KSL cells were transplanted into irradiated recipients, suggesting an HSC cell-autonomous necdin function (data not shown). Secondary transplantation assay was also performed using BM MNCs from primary recipients that received 4 × 105 BM MNCs with equal numbers of competitor cells. At 12 weeks after transplantation, BM MNCs from these recipients were pooled and transplanted into the secondary recipient mice. The chimerism of peripheral blood at 16 weeks after transplantation was almost identical in Ndn+/+ and Ndn+m/−p secondary recipients (supplemental Figure 5).
Accelerated short-term hematopoietic reconstitution by Ndn-deficient HSCs. (A) Short-term advantage in reconstitution capacity of Ndn+m/−p KSL cells in vivo. Irradiated recipient mice were transplanted with 1 × 103 KSL cells from Ndn+/+ or Ndn+m/−p mice plus 4 × 105 competitor cells in a competitive assay. Data shown are the mean percentage ± SD of donor-derived cells (total, myeloid, B-lymphoid, and T-lymphoid lineage) in the peripheral blood at the indicated times after transplantation (n = 7). *P < .05. **P < .01. (B-C) Enhanced hematologic regeneration capacity of Ndn+m/−p KSL cells. Irradiated recipient mice were transplanted with 500 KSL cells from Ndn+/+ or Ndn+m/−p mice in a noncompetitive assay. The count of (B) white blood cells (WBC) and (C) platelets in the peripheral blood of recipient mice was determined at the indicated times after transplantation. Data shown are mean number ± SD (*P < .05, **P < .01, Ndn+/+: n = 9, Ndn+m/−p: n = 10).
Accelerated short-term hematopoietic reconstitution by Ndn-deficient HSCs. (A) Short-term advantage in reconstitution capacity of Ndn+m/−p KSL cells in vivo. Irradiated recipient mice were transplanted with 1 × 103 KSL cells from Ndn+/+ or Ndn+m/−p mice plus 4 × 105 competitor cells in a competitive assay. Data shown are the mean percentage ± SD of donor-derived cells (total, myeloid, B-lymphoid, and T-lymphoid lineage) in the peripheral blood at the indicated times after transplantation (n = 7). *P < .05. **P < .01. (B-C) Enhanced hematologic regeneration capacity of Ndn+m/−p KSL cells. Irradiated recipient mice were transplanted with 500 KSL cells from Ndn+/+ or Ndn+m/−p mice in a noncompetitive assay. The count of (B) white blood cells (WBC) and (C) platelets in the peripheral blood of recipient mice was determined at the indicated times after transplantation. Data shown are mean number ± SD (*P < .05, **P < .01, Ndn+/+: n = 9, Ndn+m/−p: n = 10).
To further evaluate the difference in regenerative capacity of Ndn-deficient HSCs, 500 KSL cells from either Ndn+m/−p or Ndn+/+ mice were transplanted into lethally irradiated mice and the kinetics of the recovery of leukocyte and platelet populations measured after transplantation. The mice that received Ndn+m/−p KSL cells exhibited more rapid recovery of peripheral leukocytes and platelets than those transplanted with Ndn+/+ KSL cells (Figure 4B-C). Hence, these data indicate that short-term hematopoietic repopulating ability is selectively enhanced in Ndn-deficient HSCs. Given specific expression of necdin in HSCs, these results suggest that necdin may play a role in restricting the hematopoietic regeneration capacity of HSCs during the regeneration phase, whereas its role in steady-state hematopoiesis is rather limited.
Necdin restricts HSC proliferation during hematopoietic regeneration in response to myelosuppressive treatment
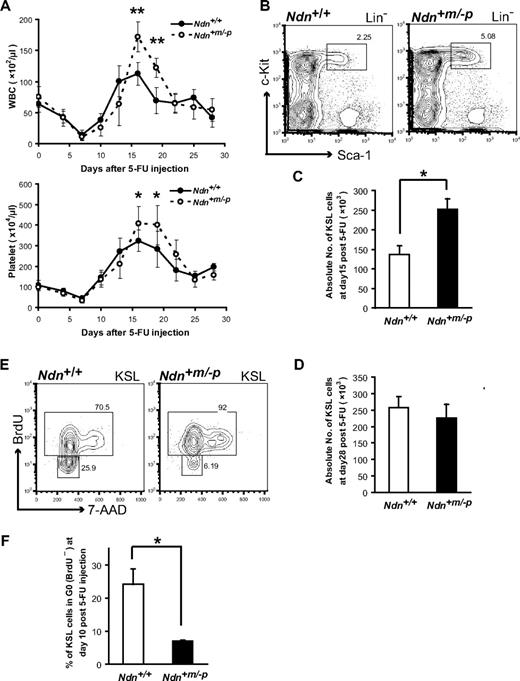
The results of competitive reconstitution assays suggest that necdin is selectively implicated in the regulation of hematopoietic regeneration after myelosuppressive injuries. To further test this in a distinct myelosuppressive condition, we treated mice with the myelotoxic agent 5-FU and examined the effect of necdin deficiency on hematologic recovery. Ndn+/+ and Ndn+m/−p mice were intravenously injected with a single dose of 5-FU (200 mg/kg), and leukocyte and platelet recoveries were monitored every third day during a total of 28 days after 5-FU treatment. Both the leukocyte and platelet counts were significantly higher in Ndn+m/−p mice than those of Ndn+/+ mice at the end of the recovery phase (days 16 and 19), with the mutant mice displaying a significant overshoot in cell numbers (Figure 5A).
Considering necdin as a potential negative cell-cycle regulator, we reasoned that the accelerated hematologic recovery in Ndn-deficient mice could be the result of an increased number of proliferating progenitor cells. In support of this notion, we found that, at day 15 after 5-FU treatment, the absolute number of KSL cells was significantly increased in Ndn+m/−p mice (Figure 5B-C). By contrast, at day 28 after treatment, the numbers of KSL cells were similar in Ndn+m/−p and Ndn+/+ mice (Figure 5D). These results suggest that the proliferation of KSL cells was transiently enhanced in Ndn-deficient mice after 5-FU treatment.
Enhanced hematopoietic recovery in Ndn-deficient mice after 5-FU treatment. (A) Kinetics of hematopoietic recovery in Ndn-deficient mice after myelosuppression with a single dose of 5-FU. Mice were intravenously injected with 5-FU (200 mg/kg), and the number of white blood cells (WBC; top panel) and platelets (bottom panel) were counted at the indicated time. Data shown are representative from 2 independent experiments that gave similar results (n = 10). Error bars represent SD. *P < .05. **P < .01. (B-D) Transient enhanced proliferation of Ndn+m/−p HSCs after 5-FU treatment. Mice were intravenously injected with 5-FU (200 mg/kg) and BM KSL cells analyzed at day 15 and day 28 after 5-FU. (B) Representative FACS profiles of BM KSL cells from Ndn+/+ and Ndn+m/−p mice at day15 after 5-FU injection. (C) The absolute number of KSL cells at day 15 (n = 3). *P = .013. (D) The absolute number of KSL cells at day 28 (n = 3). (E-F) Increased BrdU incorporation (days 7-10) in Ndn+m/−p HSCs after 5-FU treatment. Mice were intravenously injected with 5-FU (200 mg/kg); and 3 days before analysis, mice received an intraperitoneal injection of BrdU (1 mg/6 g of mouse weight) after which BrdU was included in the drinking water (1 mg/mL). BrdU incorporation in BM KSL cells was evaluated using anti-BrdU antibodies. (E) Representative FACS profile of KSL cells at day 10 after 5-FU injection. (F) Mean percentage ± SD of KSL cells in G0 (BrdU−) at day10 after 5-FU injection (n = 3). *P = .038.
Enhanced hematopoietic recovery in Ndn-deficient mice after 5-FU treatment. (A) Kinetics of hematopoietic recovery in Ndn-deficient mice after myelosuppression with a single dose of 5-FU. Mice were intravenously injected with 5-FU (200 mg/kg), and the number of white blood cells (WBC; top panel) and platelets (bottom panel) were counted at the indicated time. Data shown are representative from 2 independent experiments that gave similar results (n = 10). Error bars represent SD. *P < .05. **P < .01. (B-D) Transient enhanced proliferation of Ndn+m/−p HSCs after 5-FU treatment. Mice were intravenously injected with 5-FU (200 mg/kg) and BM KSL cells analyzed at day 15 and day 28 after 5-FU. (B) Representative FACS profiles of BM KSL cells from Ndn+/+ and Ndn+m/−p mice at day15 after 5-FU injection. (C) The absolute number of KSL cells at day 15 (n = 3). *P = .013. (D) The absolute number of KSL cells at day 28 (n = 3). (E-F) Increased BrdU incorporation (days 7-10) in Ndn+m/−p HSCs after 5-FU treatment. Mice were intravenously injected with 5-FU (200 mg/kg); and 3 days before analysis, mice received an intraperitoneal injection of BrdU (1 mg/6 g of mouse weight) after which BrdU was included in the drinking water (1 mg/mL). BrdU incorporation in BM KSL cells was evaluated using anti-BrdU antibodies. (E) Representative FACS profile of KSL cells at day 10 after 5-FU injection. (F) Mean percentage ± SD of KSL cells in G0 (BrdU−) at day10 after 5-FU injection (n = 3). *P = .038.
To examine whether necdin deficiency results in increased HSC proliferation during hematologic recovery, we performed BrdU incorporation to directly estimate the frequency of proliferating cells within the KSL population after the 5-FU treatment. Ndn+m/−p and Ndn+/+ mice injected with a single dose of 5-FU (200 mg/kg) at day 0, received a 3-day exposure to BrdU10 (days 7-10), and the proportion of KSL cells that had incorporated BrdU was measured by flow cytometry. A significant increase of BrdU-labeled KSL cells was observed in Ndn+m/−p mice at day 10 (Figure 5E-F), providing direct evidence for the acceleration of cell proliferation in KSL cells lacking Ndn. These data clearly indicate a negative regulatory function of necdin in controlling HSC proliferation.
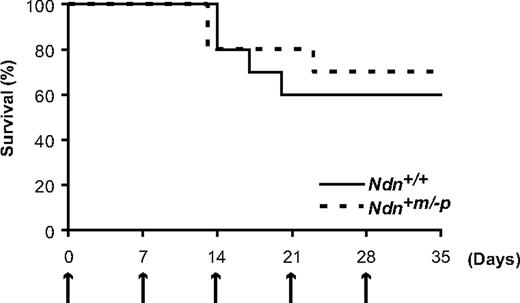
Precocious HSC exhaustion does not occur in Ndn-deficient mice after repetitive myelosuppression
It has been reported that defects in negative cell-cycle regulation of HSCs result in their precocious exhaustion after repetitive 5-FU treatment.9,12 These observations led us to investigate whether HSC exhaustion might occur in Ndn+m/−p mice under identical 5-FU treatment. For this, Ndn+/+ and Ndn+m/−p mice were given 5-FU (150 mg/kg) weekly for a total of 5 weeks and the survival of the animals followed. It has been shown that most p21-deficient and Foxo3a-deficient mice that receive an identical regimen of 5-FU die within one month because of HSC exhaustion.9,12 However, most of both Ndn+/+ and Ndn+m/−p mice survived at least 5 weeks after treatment (Figure 6). Hence, these data indicate that, despite the cell-cycle acceleration of Ndn-deficient HSCs, Ndn+m/−p mice do not exhibit HSC exhaustion under this regimen of 5-FU treatment.
HSC exhaustion does not occur in Ndn-deficient mice. Response of Ndn+m/−p mice to sequential 5-FU treatment demonstrates a normal sensitivity to repetitive myelotoxic stress. Mice were intraperitoneally injected with 5-FU (150 mg/kg) weekly, and survival was monitored daily. Survival data were analyzed by a log-rank nonparametric test and shown as Kaplan-Meier survival curves (P = .71, n = 10).
HSC exhaustion does not occur in Ndn-deficient mice. Response of Ndn+m/−p mice to sequential 5-FU treatment demonstrates a normal sensitivity to repetitive myelotoxic stress. Mice were intraperitoneally injected with 5-FU (150 mg/kg) weekly, and survival was monitored daily. Survival data were analyzed by a log-rank nonparametric test and shown as Kaplan-Meier survival curves (P = .71, n = 10).
Discussion
In the present study, we provide evidence for the role of necdin in negatively regulating HSC proliferation during hematopoietic regeneration. Consistent with our data, Liu et al previously reported, on the basis of in vitro experiments, that necdin promotes the cell-cycle exit of HSCs, thus serving to maintain their quiescence.29 However, our in vivo observation of Ndn-deficient mice reveals that this function is restricted to the regenerative phase of hematopoiesis. We find that necdin deficiency results in the transient acceleration of hematologic recovery after myelotoxic injuries, whereas no obvious abnormality is seen in steady-state hematopoiesis. This suggests that necdin preferentially functions during hematopoietic regeneration but that its role during steady-state hematopoiesis appears to be largely dispensable. As necdin deficiency has no overt effect on hematopoietic differentiation, this result suggests that accelerated hematologic recovery can be attributed to the enhanced proliferation of HSCs. Consistent with this idea, Ndn-deficient mice displayed a transient increase in the number of proliferating HSCs shortly after the myelosuppressive treatment. Thus, these data suggest a negative regulatory function of necdin in the regulation of HSC proliferation during the regenerative phase of hematopoiesis. Although we observed large differences in the numbers of proliferating HSCs during hematopoietic recovery, these translated into rather small and short-lived differences in peripheral blood components. This and the fact that we were unable to identify any apparent disadvantage associated with the loss of necdin function in HSCs make it possible that necdin, at least under the conditions tested here, does not have a significant biologic function in HSCs. However, our data indicate that necdin plays a role in the regulation of HSC proliferation; and given the highly context dependent function of necdin, it seems probable that there are circumstances under which necdin function is biologically significant.
Accumulating evidence indicates pleiotropic roles of necdin in diverse cellular processes, including cell-cycle control, survival, differentiation, and the hypoxia response. One important feature of necdin is that its biologic function is highly cell context dependent. This could be partly explained by its ability to interact with a wide variety of distinct regulatory proteins, such as Arnt2, E2f1, E2f4, Eid1, Hif1a, Msx2, Ngfr, and Trp53, dependent on the cell context.24,25,29,43-48 Although necdin itself acts as a transcriptional repressor under certain circumstances, it is probable that necdin exerts much of its function through interactions with partner proteins, thereby modulating their biologic functions, and that the context-dependent role of necdin is specified depending on the function of its partner protein. For instance, in myoblasts, necdin interferes with the Msx2-mediated repression of myogenesis to promote myogenic differentiation,47 whereas in adipocyte progenitor cells, necdin antagonizes E2f4's brown adipocyte differentiation-inducing activity.44 Similarly, the physical interaction between necdin and Trp53 results in the suppression of Trp53-induced apoptosis only in specific cell lineages.25 Necdin has also been shown to interact with Hif1a to attenuate the hypoxia response.46 Therefore, the identification of proteins interacting directly with necdin in HSCs might lead to a better understanding both of the control of HSC proliferation and the biologic role of necdin.
Similar to the negative cell-cycle regulatory function of necdin in HSCs, necdin has been implicated in inhibition of cell-cycle progression in postmitotic neurons,30 where necdin represses the transcriptional activity of E2f1 resulting in G1/G0 cell-cycle exit.24 Our gene expression profiling indicates that E2f1 is expressed at a low level in quiescent CD34−/low KSL cells but at a much higher level in actively proliferating CD34+ KSL cells (supplemental Figure 6), indicating a positive correlation between E2f1 expression and proliferation of hematopoietic progenitor cells. In support of this observation, it has recently been reported that E2f1 and E2f2 are indispensable in cell-cycle progression of hematopoietic stem/progenitor cells.49 Hence, it is tempting to speculate that necdin serves in a negative feedback loop in which necdin limits the excessive proliferation of hematopoietic stem/progenitor cells by antagonizing the E2f1-mediated HSC cell-cycle activation on myelotoxic injuries. Intriguingly, the fact that E2f1 transcription in CD34−/low KSL cells is maintained at low levels may also suggest why necdin function is negligible in steady-state HSCs. However, we cannot exclude the possibility that necdin function might be compensated by other melanoma antigen family proteins present in HSCs. More detailed biochemical analyses are required to characterize the exact molecular function of necdin in HSCs.
In conclusion, we have demonstrated that necdin plays an important role in restricting the proliferation of hematopoietic stem/progenitor cells after myelotoxic injuries, whereas during steady-state hematopoiesis necdin appears to be dispensable for the proper maintenance of HSCs. As a consequence, loss of Ndn results in an overshoot of blood recovery during the hematopoietic regeneration without affecting steady-state hematopoiesis. Our implication of necdin in hematologic recovery after myelosuppressive injuries but not in steady-state hematopoiesis suggests a potential application in novel clinical therapies. As rapid hematologic recovery is one of the key factors for successful clinical HSC transplantation, repressing necdin function could be beneficial after transplantation to achieve accelerated hematologic recovery.
Necdin deficiency has been identified as a candidate responsible for Prader-Willi syndrome, although its implications to the pathogenesis of the syndrome are incompletely understood.26-28 It has recently been reported that patients with Prader-Willi syndrome have a 40 times higher incidence of myeloid leukemia than normal persons.50 Although our Ndn-deficient mice did not develop leukemia under normal conditions, it is possible, given the elevated proliferation of HSCs in Ndn-deficient mice, that necdin deficiency might contribute to the development of leukemia. Hence, in this regard, more future studies of Ndn-deficient mice are desirable to determine whether there is any correlation between necdin deficiency and leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J. Iovanna for providing Nupr1-deficient mice, Dr Y Fujii-Kuriyama for providing Klf9 (Bteb1)-deficient mice, and the Laboratory for Animal Resources and Genetic Engineering in RIKEN CDB for the maintenance of mice.
This work was supported by in part by a Grant-in-aid for Scientific Research Priority Areas (17045037) (M.O.) and a grant for Regenerative Medicine Realization Project (S.-I.N.) from Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Authorship
Contribution: Y.K. performed the experiments and analyzed results; M.O. performed microarray analysis; L.M.J. provided informatics support; K.Y. made the Ndn-deficient mice and rabbit anti-necdin polyclonal antibody; and Y.K., M.O., and S.-I.N. designed the research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yasushi Kubota, Laboratory for Stem Cell Biology, RIKEN Center for Developmental Biology, Chuo-ku, Kobe, Hyogo 650-0047, Japan; e-mail: kubotay@cdb.riken.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal