Abstract
There is no standard therapy for steroid-refractory chronic graft-versus-host disease (GVHD). This problem is particularly daunting in children with chronic GVHD, whereby the effects of the disease and its treatment may impair normal growth and development. Children are also particularly vulnerable to failure and/or toxicity of therapy; for example, joint contractures or joint damage may result in life-long disability. The Pediatric Blood and Marrow Transplant Consortium performed a phase 2 trial of pentostatin for steroid-refractory chronic GVHD in 51 children (median age, 9.8 years) from 24 institutions. Overall response was 53% (95% confidence interval, 40%-64%), with a response of 59% (95% confidence interval, 42%-75%) in sclerosis. Thirteen subjects (25%) had toxicity requiring them to stop pentostatin. The drug had a significant steroid-sparing effect in those that responded. A trend was also observed toward increased survival at 3 years in responders versus nonresponders (69% vs 50%; P = .06). The intravenous administration of the drug ensures compliance in a patient group in which oral therapy is difficult to monitor. Pentostatin has activity in refractory chronic GVHD in children, and future studies, including treatment of children newly diagnosed with high-risk chronic GVHD, are warranted. The trial was registered at www.Clinicaltrials.gov as #NCT00144430.
Introduction
Chronic graft-versus-host disease (GVHD) is the main cause of late morbidity and non–relapse mortality after hematopoietic stem cell transplantation (HSCT). Pediatric chronic GVHD in particular remains an understudied area. The recovery of the pediatric immune system after HSCT is different from that of adults,1 and the stem cell source use in pediatrics is clearly different (eg, more cord blood and less peripheral blood than in adults). Consequently, response to immunosuppression may not be the same in children. Furthermore, children have many years to live after HSCT; therefore, minimizing irreversible changes of chronic GVHD, such as joint contractures or pulmonary fibrosis, is of paramount importance. The toxicity of long-term corticosteroid therapy is also a significant problem particularly for pediatric patients for whom the effects on bone and growth and development are more pronounced. Thus, there is a need for corticosteroid-sparing therapy in this group of patients.
Unfortunately, therapy for chronic GVHD has been associated with suboptimal responses and significant toxicity and morbidity. Systemic corticosteroids with or without a calcineurin inhibitor remain the standard for initial therapy.2,3 However, many patients do not respond adequately and need further treatment. Recently reported salvage therapies include sirolimus,4 mycophenolate mofetil (MMF),5 rituximab,6 and extracorporeal photopheresis (ECP).7,8 Salvage studies in pediatric chronic GVHD have been small and limited mostly to MMF9 and ECP.10,11 MMF showed minimal response in what is considered a very difficult type of chronic GVHD to treat: sclerotic skin GVHD.9 Sclerotic GVHD in children has shown some response to ECP10,11 ; however, it appears that this therapy is needed for a prolonged period of time to see an effect.
Furthermore, a number of these therapies have significant toxicities that can limit their utility, such as hemolytic uremic syndrome seen with sirolimus4 and significant diarrhea seen with MMF.9 Some therapies are more difficult in children. For example, ECP requires significant supportive care such as prolonged central venous line access and multiple transfusions throughout the length of treatment.10,11 Although compliance can be an issue with any age group, oral therapy can be particularly problematic for some children and young adults. Clearly, in a disease such as chronic GVHD there is need for a well-tolerated, easily administered and monitored therapy.
Pentostatin, a nucleoside analog that is a potent inhibitor of adenosine deaminase,12 has a broad spectrum of immunomodulatory activities. Most relevant to GVHD, this drug causes marked reduction of CD4 and CD8 cells. There is also significant B-cell depletion with reduction of IgG levels.13 This should allow it to affect GVHD at the cellular level and thus has the potential to address the many manifestations of chronic GVHD. This is in contrast to other agents, such as imatinib,14 which targets selective pathways involved in fibrosis, and rituximab,6 which targets antibody production. Pentostatin was found to be active in a phase 1 study in refractory acute GVHD.15 A phase 2 study of pentostatin in heavily pretreated patients (median age, 33 years; median of 4 prior regimens) with chronic GVHD showed a 55% objective response rate in 58 patients.16
Although responses to pentostatin in an adult population are very encouraging, that study was conducted at only 2 centers in a predominantly adult population. Because response in pediatric patients could theoretically be different from that in older populations, we conducted a multi-institutional study of pentostatin (deoxycoformycin [Nipent]; Hospira Inc) in pediatric patients with corticosteroid-refractory chronic GVHD to look at response rate and to detect any unique toxicities in this age group.
Methods
Eligibility and enrollment
For all subjects, parental permission and subject assent, when applicable, were obtained in accordance with the Declaration of Helsinki. The study was approved by the institutional review boards of all participating Pediatric Blood and Marrow Transplant Consortium (PBMTC) institutions. The trial was registered at www.clinicaltrials.gov with identifier NCT00144430 on August 31, 2005.
To be eligible for study participation, subjects were required to have had an allogeneic stem cell transplant using any stem cell source and to be less younger than 21 years. In addition, subjects were required to have treatment-refractory chronic GVHD, defined as (1) development of 1 or more new sites of disease while being treated for chronic GVHD, (2) progression of existing sites of disease while receiving treatment for chronic GVHD, or (3) failure to improve despite at least 1 month of standard treatment for chronic GVHD. Standard therapy was defined as a regimen containing at least 1 mg predinsone/kg every other day or equivalent dose of another steroid or another immunosuppressive regimen if the patient was unable to tolerate steroid therapy. Because eligibility was based on clinical criteria of chronic GVHD, subjects did not need to be more than 100 days after HSCT to be eligible. A tissue biopsy before entering the study with histology consistent with GVHD was required unless there was a medical contraindication such as concern for poor wound healing after the biopsy.
There were no restrictions about the preparative regimen received or the degree of human leukocyte antigen (HLA) mismatching. Myeloablative regimens were defined as those including at least 14 mg busulfan/kg or total body irradiation of at least 1200 cGy before transplantation. Reduced intensity included all others. HLA typing reported was for HLA-A, HLA-B, and HLA-DRB1.
Patients who had failed more than 2 prior immunosuppressive regimens (in addition to their GVHD prophylaxis regimen) were not eligible. Patients with a forced expiratory volume in 1 second less than 50% were also not eligible. Patients with a Karnofsky/Lansky performance score less than 40% were excluded, as were those with a calculated creatinine clearance of less than 30 mL/min per 1.73 m2.
Therapy
Pentostatin was the only study intervention for the treatment of chronic GVHD for participants. Pentostatin was administered every 2 weeks at 4 mg/m2 by intravenous infusion over 20 to 30 minutes. An intravenous fluid bolus (5 mL/kg) was given before and after each dose. Dose was modified as follows: if the absolute neutrophil count was below 0.5 × 109/L (500 cells/mm3) or the platelet count was below 20 × 109/L (20 000/mm3) or the estimated creatinine clearance was less than 50 mL/min per 1.73 m2 or greater than 30 mL/min per 1.73m2, the dose was reduced by 50%. If the estimated creatinine clearance was less than 30 mL/min per 1.73 m2, pentostatin was held. Pentostatin was also held during severe infections at the discretion of the local principal investigator (PI). If subjects sustained a complete response at 6 months, pentostatin administration was stopped, and subjects were followed for an additional 6 months to determine sustained response. All other subjects received 12 months of therapy unless they required earlier removal from the study.
It was recommended that a corticosteroid taper be started between 8 and 12 weeks after initiating pentostatin. A reduction of 25% of the initial dose every 2 weeks was the recommended corticosteroid taper. It was also recommended that if subjects were receiving a calcineurin inhibitor at the time of study initiation that they remain on it through the duration of the study. All other immunosuppressants were to be tapered, starting at 3 months.
The following infection prophylaxis was strongly recommended, but not required, for subjects on study: trimethoprim-sulfamethoxazole for Pneumocystis jiroveci prophylaxis, penicillin for antibacterial prophylaxis, acyclovir for antiviral prophylaxis, and fluconazole for antifungal prophylaxis.
Required evaluations and clinical endpoints
Results of skin biopsies and/or additional pertinent organ biopsies were reviewed by the PI. At baseline and every 2 weeks, a history and physical were required, as well as complete blood count, chemistries, and liver function tests. A calculated creatinine clearance using the Schwartz formula was determined before each dose of pentostatin. Schirmer tests and pulmonary function tests were performed at baseline if the subject was old enough to cooperate and throughout the study as needed. Additional biopsies were performed when clinically indicated.
Subjects were evaluated at baseline and then every 3 months, using the form in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The staging used in this study was very similar to the Children's Oncology Group ASCT0031 response criteria and the National Institutes of Health (NIH) Consensus for Chronic GVHD staging recommendations, which were not yet available at the time this study started.17 Subjects were graded in 9 domains. These domains combined reported symptoms and physical findings, on a scale from 0 to 3. Subjects were graded by each center's investigator or designee. In addition, a detailed symptom list and medication reports were submitted every 3 months. Domain responses were then determined based on the grading form. A total of 9 domains were assessed: rash, sclerosis, oral, fasciitis, joint, liver, lung, gastrointestinal, and ocular.
To obtain a response in a particular domain, the following criteria were used: For a partial response, there must be improvement in at least 1 of the items without worsening in any other item (ie, for “rash” response a subject needed improvement in “rash” and/or “lichenoid changes” without worsening in other domains). For a complete domain response, a subject must be graded as zero in all items in that domain at the subject's last evaluation. Finally, a complete overall response was obtained by having all involved domains reach a complete response at the subject's final evaluation, whereas a partial overall response represented partial response in any domain(s) with no worsening in any other. No response was defined as progression, no change, or mixed response (improvement in 1 domain and worsening in another). According to the protocol, possibly irreversible manifestations (hypopigmentation/hyperpigmentation and ocular GVHD) were not used in determining overall response. Because of the subjective nature of ocular dryness, a score of 0 or 1 represented a complete ocular response, in a subject beginning with a score of 2 or 3. All subjects, regardless of number of pentostatin doses received, were evaluable for response. Overall response and organ-specific response were determined from the subject's last evaluation, even if the subject was removed from the study early (ie, before 12 months) for toxicity, progression of chronic GVHD, or decision of the local PI or subject. Patients having an initial response and then a subsequent progression were considered nonresponders. Thus, the overall response represents the best response except as stated above (response and subsequent progression was considered a nonresponse). Subjects were not followed for response beyond the completion of the study (12 months).
Toxicity sheets were filled out on a monthly basis using Common Toxicity Criteria Version 3.0. Nonhematologic toxicities of at least grade 3 were reported in an expedited fashion. The reporting period for adverse events and toxicities was 28 days after the last on-study dose.
Removal from protocol
Subjects were removed from the study if they experienced disease progression after 3 doses of pentostatin or if they experienced a grade 3 to 4 toxicity (related or not related to pentostatin) that was judged serious enough to preclude the subject from receiving further administration of the drug. Subjects were also removed if they were lost to follow-up or if the family withdrew consent. For subjects that were removed from the study, the last assessment before removal was used to determine response. Subjects that were removed before the first 3-month evaluation for either toxicity or progression, and in whom a final assessment was not filled out (n = 8), are counted as nonresponders.
Consensus review, statistical considerations, and safety monitoring
A panel of 6 investigators (D.A.J., K.T., E.R.N., M.G., L.L., and A.L.G.) convened for 2 days at the end of the study for response adjudication. Each member of the panel individually reviewed all case report forms and severe adverse events for every subject. Domain response, overall response, and attribution of severe adverse events were recorded by each member, and then each subject was reviewed by the panel. With regards to overall response, there was unanimous agreement in 84% of cases and agreement by 5 of the 6 experts in another 10% of cases. In the remaining 6% of cases, consensus was reached after discussion by the panel.
The primary statistical endpoint was overall response rate (defined as complete or partial remission). A Simon 2-stage design was used to have 80% power to detect a response rate of at least 40%18 compared with a control response rate of 20%, with 80% power and a type I error rate of 5%. Although this design required 43 patients, a sample size of 50 was targeted, whereas the final sample size was 51. The other main outcome of the study was determination of adverse events.
A secondary outcome was response in each domain, measured in subjects that had had initial involvement in that domain. Response rates were calculated together with 95% exact binomial confidence intervals. Descriptive characteristics are shown with percentages or with medians and ranges, as appropriate. Fisher exact test was used to determine significance (judged as P < .05) of certain factors such as change in corticosteroid dose and effect of early response on final response. Time to initial response was analyzed using cumulative incidence when withdrawal because of toxicity was considered a competing event.19 Nonresponders who were not withdrawn because of toxicity were considered to be censored at their last follow-up. Survival percentages and confidence intervals were calculated using Kaplan-Meier curves. Curves were compared using the log-rank test. All analyses were performed with SAS (SAS Institute).20
This study was overseen by the PBMTC Data Safety Monitoring Committee. Study reports were reviewed twice yearly. Reports included response and toxicity data. The study was designed to stop if it was determined early that it was futile treatment (if 3 or fewer of the first 13 subjects did not respond to treatment). The study continued to full accrual. The study was also overseen by the Food and Drug Administration under IND #67084.
Results
Patient characteristics
Fifty-one subjects from 24 different institutions were enrolled. The characteristics before transplantation of the 51 subjects enrolled are shown in Table 1. Briefly, the median age at time of enrollment was 9.8 years (range, 0.9-20.7 years). Thirty-one subjects had received a HSC transplant for a malignant disease. Forty of the subjects had received an alternative donor stem cell source: unrelated cord blood (n = 18), adult unrelated donor (n = 19), and nongenotypically matched family member donor (n = 3). Subjects had had chronic GVHD for a median of 6.2 months (range, 0.1-42.1 months) before enrollment. Forty-seven of the subjects had had a tissue biopsy consistent with chronic GVHD, and in 4 of the subjects the biopsy was not performed because of medical contraindication.
Patient characteristics at study entry (N = 51)
| . | Values . |
|---|---|
| Median age, y (range) | 9.8 (0.9-20.7) |
| Median time from transplantation to GVHD, mo (range) | 5.0 (1.4-26.1) |
| Median time from GVHD to study entry, mo (range) | 6.2 (0.1-42.1) |
| Median time from transplantation to study entry, mo (range) | 12.2 (3.4-47.7) |
| Sex | |
| Male, n (%) | 27 (53) |
| Female, n (%) | 24 (47) |
| Diagnoses | |
| Acute myeloid leukemia/myelodysplasia, n (%) | 13 (25) |
| Chronic myelogenous leukemia, n (%) | 3 (6) |
| Acute lymphoblastic leukemia, n (%) | 15 (29) |
| Metabolic disorder, n (%) | 6 (12) |
| Hemoglobinopathy, n (%) | 3 (6) |
| Immune deficiency, n (%) | 1 (2) |
| Bone marrow failure syndrome, n (%) | 8 (16) |
| Familial hematophagocytic lymphohistiocytosis, n (%) | 2 (4) |
| Transplantation regimen | |
| Myeloablative, n (%) | 37 (73) |
| Reduced intensity/reduced toxicity, n (%) | 14 (27) |
| Stem cell source | |
| HLA-identical sibling bone marrow, n (%) | 3 (6) |
| HLA-identical sibling PBSC, n (%) | 7 (14) |
| HLA-identical sibling cord blood, n (%) | 1 (2) |
| Unrelated cord blood, n (%)* | 18 (35) |
| Unrelated donor bone marrow, n (%)† | 9 (17) |
| Unrelated donor PBSC, n (%)‡ | 10 (20) |
| Nongenotypically matched family member donor bone marrow (HLA 6:6), n (%) | 1 (2) |
| Nongenotypically matched family member donor PBSC, n (%)§ | 2 (4) |
| . | Values . |
|---|---|
| Median age, y (range) | 9.8 (0.9-20.7) |
| Median time from transplantation to GVHD, mo (range) | 5.0 (1.4-26.1) |
| Median time from GVHD to study entry, mo (range) | 6.2 (0.1-42.1) |
| Median time from transplantation to study entry, mo (range) | 12.2 (3.4-47.7) |
| Sex | |
| Male, n (%) | 27 (53) |
| Female, n (%) | 24 (47) |
| Diagnoses | |
| Acute myeloid leukemia/myelodysplasia, n (%) | 13 (25) |
| Chronic myelogenous leukemia, n (%) | 3 (6) |
| Acute lymphoblastic leukemia, n (%) | 15 (29) |
| Metabolic disorder, n (%) | 6 (12) |
| Hemoglobinopathy, n (%) | 3 (6) |
| Immune deficiency, n (%) | 1 (2) |
| Bone marrow failure syndrome, n (%) | 8 (16) |
| Familial hematophagocytic lymphohistiocytosis, n (%) | 2 (4) |
| Transplantation regimen | |
| Myeloablative, n (%) | 37 (73) |
| Reduced intensity/reduced toxicity, n (%) | 14 (27) |
| Stem cell source | |
| HLA-identical sibling bone marrow, n (%) | 3 (6) |
| HLA-identical sibling PBSC, n (%) | 7 (14) |
| HLA-identical sibling cord blood, n (%) | 1 (2) |
| Unrelated cord blood, n (%)* | 18 (35) |
| Unrelated donor bone marrow, n (%)† | 9 (17) |
| Unrelated donor PBSC, n (%)‡ | 10 (20) |
| Nongenotypically matched family member donor bone marrow (HLA 6:6), n (%) | 1 (2) |
| Nongenotypically matched family member donor PBSC, n (%)§ | 2 (4) |
GVHD indicates graft-versus-host disease; HLA, human leukocyte antigen; and PBSC, peripheral blood stem cell.
HLA-matching: HLA 4:6 (N = 5), HLA 5:6 (N = 11), HLA 6:6 (N = 2).
HLA- matching: HLA 5:6 (N = 1), HLA 6:6 (N = 8).
HLA- matching: HLA 5:6 (N = 4), HLA 6:6 (N = 6).
HLA-matching: HLA 5:6 (N = 1), HLA 6:6 (N = 1).
The chronic GVHD characteristics of the subjects are given in Table 2. Sixty-nine percent of subjects had had a history of prior acute GVHD, 34% had progressive-onset chronic GVHD (while on therapy for acute GVHD, they had progressed to chronic GVHD), and 59% had severe chronic GVHD according to the NIH Consensus global severity stage.17 Subjects had a variety of chronic GVHD manifestations at study entry (Table 3), with skin being the most common (78% lichenoid changes/rash and 53% sclerosis). Most of the subjects with gastrointestinal tract manifestations had additional manifestations suggestive of chronic GVHD. However, 4 subjects had gastrointestinal tract symptoms as their main GVHD manifestations, which could represent the late acute variant of GVHD.17 All subjects had received corticosteroids for treatment of chronic GVHD (median, 3.9 months; range, 1-24.9 months), and 49 were on corticosteroids at study entry, with a median prednisone dose of 0.9 mg/kg per day (range, 0-2.4 mg/kg per day). Forty-five subjects had been on a calcineurin inhibitor, and 42 of them were on one at the time of study entry. Other therapies given before this study specifically for chronic GVHD included MMF (n = 30), infliximab (n = 4), sirolimus (n = 3), daclizumab (n = 3), etanercept (n = 2), photopheresis (n = 1), and hydroxychloroquine (or placebo) on Children's Oncology Group ASCT0031, a study from the Children's Oncology Group open at that time (n = 2).
Chronic GVHD characteristics at study entry
| . | Values . |
|---|---|
| Median beginning platelet count, ×109/L (range) | 267 (16-683) |
| Median beginning prednisone dose, mg/kg/d (range) | 0.9 (0-2.4) |
| Median time on corticosteroids before entry, mo (range) | 3.9 (0-24.9) |
| Subjects on corticosteroids at study entry, n (%) | 49 (96) |
| Subjects with platelet count less than 100 × 109/L, n (%) | 10 (20) |
| Performance score no greater than 80, n (%) | 19 (37) |
| History of acute GVHD, n (%) | 35 (69) |
| Progressive onset chronic GVHD, n (%) | 17 (34) |
| Severe chronic GVHD (NIH global severity stage), n (%) | 30 (59) |
| Tissue biopsy consistent with GVHD, n (%) | 47 (92) |
| . | Values . |
|---|---|
| Median beginning platelet count, ×109/L (range) | 267 (16-683) |
| Median beginning prednisone dose, mg/kg/d (range) | 0.9 (0-2.4) |
| Median time on corticosteroids before entry, mo (range) | 3.9 (0-24.9) |
| Subjects on corticosteroids at study entry, n (%) | 49 (96) |
| Subjects with platelet count less than 100 × 109/L, n (%) | 10 (20) |
| Performance score no greater than 80, n (%) | 19 (37) |
| History of acute GVHD, n (%) | 35 (69) |
| Progressive onset chronic GVHD, n (%) | 17 (34) |
| Severe chronic GVHD (NIH global severity stage), n (%) | 30 (59) |
| Tissue biopsy consistent with GVHD, n (%) | 47 (92) |
GVHD indicates graft-versus-host disease; and NIH, National Institutes of Health.
Response by domain (N = 51)
| . | Involved, n (%) . | Complete, n (%) . | Partial, n (%) . | Stable, n (%) . | Worse, n (%) . | Response rate, % (95% CI)* . |
|---|---|---|---|---|---|---|
| Rash/lichenoid changes | 40 (78) | 13 (33) | 7 (17) | 10 (25) | 10 (25) | 50 (36-64) |
| Sclerosis | 27 (53) | 11 (41) | 5 (18) | 6 (22) | 5 (19) | 59 (42-75) |
| Oral | 30 (59) | 9 (30) | 8 (27) | 7 (23) | 6 (20) | 57 (40-72) |
| Fasciitis | 7 (14) | 2 (29) | 1 (14) | 2 (29) | 2 (29) | 43 (18-71) |
| Joint | 20 (39) | 5 (25) | 4 (20) | 5 (25) | 6 (30) | 45 (27-64) |
| Liver | 5 (10) | 0 | 0 | 2 | 3 | 0 |
| Lung | 2 (4) | 0 | 0 | 1 | 1 | 0 |
| Gastrointestinal | 21 (41) | 8 (38) | 1 (5) | 7 (33) | 5 (24) | 43 (25-62) |
| Ocular | 28 (55) | 5 (18) | 2 (7) | 13 (46) | 8 (29) | 25 (13-41) |
| . | Involved, n (%) . | Complete, n (%) . | Partial, n (%) . | Stable, n (%) . | Worse, n (%) . | Response rate, % (95% CI)* . |
|---|---|---|---|---|---|---|
| Rash/lichenoid changes | 40 (78) | 13 (33) | 7 (17) | 10 (25) | 10 (25) | 50 (36-64) |
| Sclerosis | 27 (53) | 11 (41) | 5 (18) | 6 (22) | 5 (19) | 59 (42-75) |
| Oral | 30 (59) | 9 (30) | 8 (27) | 7 (23) | 6 (20) | 57 (40-72) |
| Fasciitis | 7 (14) | 2 (29) | 1 (14) | 2 (29) | 2 (29) | 43 (18-71) |
| Joint | 20 (39) | 5 (25) | 4 (20) | 5 (25) | 6 (30) | 45 (27-64) |
| Liver | 5 (10) | 0 | 0 | 2 | 3 | 0 |
| Lung | 2 (4) | 0 | 0 | 1 | 1 | 0 |
| Gastrointestinal | 21 (41) | 8 (38) | 1 (5) | 7 (33) | 5 (24) | 43 (25-62) |
| Ocular | 28 (55) | 5 (18) | 2 (7) | 13 (46) | 8 (29) | 25 (13-41) |
CI indicates confidence interval.
Response rate included CR + PR.
Response
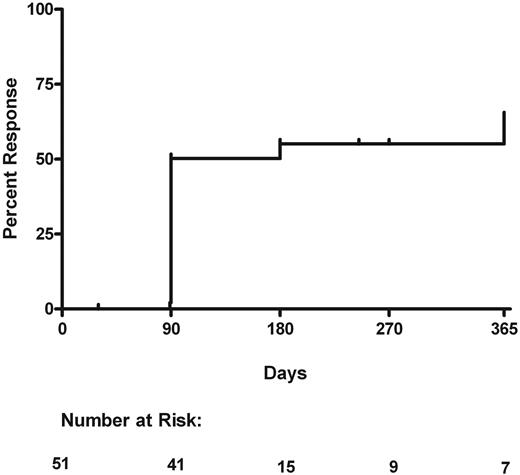
Overall, 27 subjects had a response to treatment at their last evaluation, for an overall response rate of 53% (95% confidence interval [CI], 40%-64%). Twenty subjects had a partial response, and 7 had a complete response. These 27 subjects had an initial documented response as follows: 23 at 90 days, 2 at 180 days, and 2 at 365 days. All of these 27 subjects sustained their response at time of last evaluation, which was at the same time of initial response in 5 patients, at a range of 3 to 185 days after initial response in 9 patients and 275 days after initial response in 13 patients. The median duration of response was 185 days (range, 0-275 days) after their initial response. For 20 subjects the initial response remained the best response; however, 7 continued improving during their course of treatment. The last evaluation times were at 90 to 285 days in 11 responders and at 365 days in 16 responders. The median follow-up time in all 51 patients was 270 days (range, 25-365 days). Twenty-four patients were nonresponders. Of these, 15 never showed a response, and 9 subjects had an initial or transient response but later progressed. The cumulative incidence of time to initial response when withdrawal because of toxicity is a competing risk is shown in Figure 1.
Cumulative incidence of initial response. Withdrawal because of toxicity is considered a competing event. Number at risk indicates the number of patients still on study at that time point who have either (1) not had an initial response or (2) not been withdrawn for toxicity or (3) not been censored.
Cumulative incidence of initial response. Withdrawal because of toxicity is considered a competing event. Number at risk indicates the number of patients still on study at that time point who have either (1) not had an initial response or (2) not been withdrawn for toxicity or (3) not been censored.
Of the 51 subjects who entered the study, 21 received the intended 12 months of therapy. Removal from study before 12 months occurred for the following reasons: chronic GVHD progression (13), toxicity (13), relapse of malignancy (2), and loss to follow-up (2). Of the 21 subjects that received 12 months of therapy, 16 had an objective response (10 partial response, 6 complete response). Using the total sample size as the denominator, the percentage of patients who received 12 months of therapy and had a response was 16/51 (31%; 95% CI, 20%-44%).
As for organ-specific response, among the 40 subjects with skin rash/lichenoid involvement, the response rate was 50% (95% CI, 36%-64%). Of the 27 subjects with sclerotic manifestations, 59% (95% CI, 42%-75%) showed response. All other manifestations and responses are detailed in Table 3. No subjects with liver (n = 5) or lung (n = 2) manifestations had a response in these domains. Of the 5 subjects with liver GVHD, 3 were overall nonresponders (2 of these had skin GVHD that worsened; 1 had liver GVHD as the only manifestation). One subject with liver GVHD was stable overall (liver GVHD was the only manifestation). The last subject with liver GVHD was an overall responder (the liver remained stable and rash/sclerosis improved). Of the 2 subjects with lung manifestations, 1 had stable lung involvement with improvement in rash and was classified as a responder, whereas the other had worsening lung function with improvement in rash and was classified as a nonresponder.
As secondary evaluations, we were interested in determining whether early response predicted overall response and ability to continue on the study. There were 29 early responders (response by 3 months). Thirteen of those (45%) continued on the study and maintained a partial or complete response at 12 months. There were 22 patients who were not considered responders at the 3-month evaluation. Because the tempo of response was not known, some of these patients continued on the study at the discretion of the investigator. Three (14%) of these 22 patients continued on the study and achieved a partial (n = 2, initial response in both patients at 6 months) or complete response (n = 1, response at 12 months; P = .031 comparing 45% with 14%). Furthermore, we were interested in determining whether pentostatin provided a corticosteroid-sparing effect in subjects that responded. The initial median dose of prednisone in responding patients was 0.9 mg/kg per day (range, 0-2.4 mg/kg per day) and at study end it was 0.2 mg/kg per day (range, 0-1.1 mg/kg per day; P < .001). In the 24 subjects who did not respond to pentostatin, the initial median prednisone dose was 0.8 mg/kg per day (range, 0-2.2 mg/kg per day). Final dose was available for 15 of these nonresponding subjects, and it was 0.9 mg/kg per day (range, 0-2.2 mg/kg per day). The change in prednisone dose in nonresponding subjects was not statistically significant (P = .89).
Toxicity and mortality
Thirteen subjects went off the study because of toxicity. The toxicities in these 13 subjects are summarized in Table 4. Most adverse events occurred in the first 6 months. In fact, 11 of 13 patients discontinuing because of toxicity did so during the first 6 months of therapy. The most common adverse event was infection (n = 27), which occurred in 15 subjects. Three subjects had at least 3 infectious adverse events each, and these accounted for 13 of the infectious adverse events. There were 2 cases of documented fungal infections and 3 cases of documented viral infections. The rest were documented or presumed bacterial infections requiring hospitalizations. All 3 subjects who died of infectious complications had sepsis with documented bacterial infections (methicillin-resistant Staphylococcus aureus, Stenotrophomonas maltophilia, and Pseudomonas aeruginosa). Autoimmune hemolytic anemia was seen in 3 patients. All 3 subjects were removed from the study. With regard to malignancies, 2 subjects (of 31 at risk) experienced relapse of their primary disease (leukemia). One subject developed Epstein-Barr virus posttransplant lymphoproliferative disease.
Adverse events that required subjects to go off study
| . | Months on study . | |
|---|---|---|
| 0-6, no. of subjects . | 6-12, no. of subjects . | |
| Infections | ||
| Documented bacterial requiring infection | 2 | — |
| Presumed bacterial infection | 1 | — |
| Documented fungal infection | — | 1 |
| Documented viral infection | 1 | — |
| Central nervous system (leukoencephalopathy) | 1 | — |
| Renal | 2 | — |
| Gastrointestinal/liver (pancreatitis/abdominal pain) | 1 | — |
| Hematologic(autoimmune hemolytic anemia) | 2 | 1 |
| Allergic reaction | 1 | — |
| . | Months on study . | |
|---|---|---|
| 0-6, no. of subjects . | 6-12, no. of subjects . | |
| Infections | ||
| Documented bacterial requiring infection | 2 | — |
| Presumed bacterial infection | 1 | — |
| Documented fungal infection | — | 1 |
| Documented viral infection | 1 | — |
| Central nervous system (leukoencephalopathy) | 1 | — |
| Renal | 2 | — |
| Gastrointestinal/liver (pancreatitis/abdominal pain) | 1 | — |
| Hematologic(autoimmune hemolytic anemia) | 2 | 1 |
| Allergic reaction | 1 | — |
Subjects with adverse events that required them to be removed from study, separated by two 6-month time periods on the study.
— indicates no event.
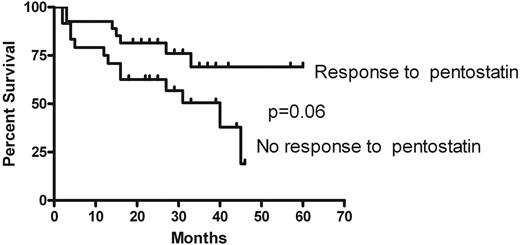
Four subjects died while on therapy or within 28 days after the last dose of pentostatin. Three of these deaths were due to infection, and 1 was attributed to leukoencephalopathy in a subject with reversible leukoencephalopathy secondary to tacrolimus that was previously diagnosed. At 1 year, the overall survival of the entire cohort was 84% (95% CI, 74%-94%). At 3 years, the projected overall survival of the cohort was 60% (95% CI, 45%-75%). At 3 years after entry on the trial, projected survival was 69% (95% CI, 49%-89%) for patients who responded to pentostatin compared with 50% (95% CI, 29%-71%) for those not responding (P = .06), as shown in Figure 2. The median follow-up of survivors was 33 months (range, 18-60 months).
Overall survival. Individual curves shown for subjects that had response compared with those that did not.
Overall survival. Individual curves shown for subjects that had response compared with those that did not.
Discussion
In this phase 2 study we examined the response and toxicity of pentostatin in children with corticosteroid-refractory chronic GVHD. The study met its primary statistical endpoint, which was to show a response rate greater than 40%.
This study is important for a number of reasons. First of all, results in a pediatric population confirm the results seen in a prior phase 2 study of mostly adult subjects.16 The prior phase 2 study was performed at 2 sites and was thus subject to potential bias of limited-institution studies. Here, 51 subjects were accrued from 24 sites, and an overall response of 53% was seen. The subjects in the current study represented a more homogenous population in terms of age and pretreatment characteristics.
Secondly, although several studies have reported responses in this range in the salvage setting, most of the skin responses have been in the lichenoid/rash domains.9,18 A remarkable finding in our study is that we documented a 59% response rate in sclerotic manifestations. Of the 9 subjects that started with 10% to 50% sclerosis by BSA, 7 had an overall response, and, of the 3 that started with greater than 50%, 2 had an overall response. Sclerosis has been a manifestation of chronic GVHD that generally is less responsive to therapy and, if it does improve, tends to take a longer time compared with other manifestations.21
In addition, we were also able to examine the tempo of response in this study and showed that those subjects with a good response at 3 months were much more likely to continue improving and to tolerate therapy for a longer period of time. This observation has important practical implications because one may consider not continuing therapy with pentostatin after 3 months if there has not been an objective response by that point. Early response seems to be the most important predictor of overall outcome because we were unable to detect a difference in response rate based on multiple baseline patient characteristics such as steroid dose and platelet count (data not shown).
Finally, this multi-institutional study is the largest reported pediatric chronic GVHD clinical trial to date. The PBMTC brings together investigators from 70 North American institutions with an interest in clinical trials in pediatric hematopoietic cell transplantation. The enthusiastic completion of this study in a timely fashion shows the feasibility of finishing studies of this size through the PBMTC and sets the stage for further studies in GVHD and supportive care after transplantation.
There is also tremendous interest at this point in designing tools for staging and assessing response of chronic GVHD. The NIH Consensus Development Project on Criteria for Clinical Trials in Chronic GVHD initially convened in 2005, and since then there have been recommendations on what criteria to use in diagnosis, staging, and response.17,22 An ongoing cohort study is prospectively studying the validity of these staging and response criteria (Dr Stephanie Lee, Fred Hutchinson Cancer Research Center, written communication, December 2007), and several retrospective studies have looked at the applicability of these scales.23-25 The staging used in this study is very close to the NIH Consensus for Chronic GVHD staging recommendations.17 Although the NIH Consensus recommends a different scale to measure response, we have shown within this study that the staging criteria are feasible to use in a multi-institutional setting and that response in this scale is consistent among a panel of graders. There was full agreement on response from the panel of graders in most of the cases. Among the 8 cases in which no full agreement was reached, in 5 of the cases the majority graded the subjects as responders and in 3 of the cases the majority graded the subjects as nonresponders. Consensus was achieved with discussion, and in all 8 cases it was the majority's assessment of response which dictated the final response assessment. Response was associated with objective, measurable, and clinically relevant endpoints. For example, response in this scale was associated with reduction in immunosuppression, now considered one important endpoint in chronic GVHD studies.26,27
Of course, one of the concerns in using a scale as we did, which is semiquantitative and combines signs and symptoms, is that it may not be sensitive enough to detect all responses. It may not have the same level of correlation with response in a group of patients with either a very high or a very low burden of GVHD signs and symptoms. In addition, the scale may not be completely applicable in an older population. Hence, the NIH Consensus has recommended a more quantitative scale, for patients of all ages, to measure response.22 It is imperative that this scale be validated, an effort which is currently under way in the ongoing cohort study described earlier. In addition, validating quality-of-life and symptom scales in pediatric patients with chronic GVHD would add additional ways with which to measure response.
We show that, along with a documented objective response and corticosteroid reduction, pentostatin administration was simple and well tolerated. The medication is administered every 2 weeks in the span of 2 to 3 hours. Short-term side effects were mostly nausea, and subjects were able to go back to their regular activities often the evening after drug administration. One of the benefits of a drug with such a long pharmacodynamic effect is this ability to dose it infrequently yet continue to have a prolonged benefit. In chronic diseases in which there is already a high burden of emotional and psychosocial issues, it would appear that simple therapies that require minimal efforts at home would be of some benefit. With an intravenous medication, monitoring of therapy is straightforward.
Although this was not a randomized study, the toxicity encountered in this population does not appear to be disproportionate to what is seen with other therapies for patients with corticosteroid-refractory chronic GVHD.4,8 For example, the recently published experience with imatinib had a similar rate of removal from study for toxicity (7/33 = 21%)14,28 as with this study (13/51 = 25%). Most of the toxicities in the current trial occurred early and were infectious in origin. It is hard to know if the infections were due to the underlying disorder (the immunodeficiency of chronic GVHD), the cumulative toxicity of prior therapies, and/or the experimental therapy being tested. Baseline factors were not significantly associated with infectious adverse events (data not shown). However, there was a trend toward more infectious adverse events in subjects with a history of acute GVHD (P = .06). With respect to other studies, most reports of salvage approaches are either too small or target a very specific patient type which makes a comparison of infectious mortality difficult. The infectious mortality (3 of 51) encountered in our study does not appear to be significantly different to what was seen in the randomized study in which MMF was added to standard therapy in patients newly diagnosed with chronic GVHD. In that study, 3 of 74 subjects on the MMF arm died of infection.29
One toxicity deserving mention is autoimmune hemolytic anemia that occurred in 3 subjects and was severe enough to require the subjects to go off study and receive treatment with rituximab. Autoimmune hemolytic anemia after HSCT has been described both in adults and in children,30,31 and one can hypothesize that the cause is the immune dysregulation from chronic GVHD, from the therapy for chronic GVHD, or both. Future chronic GVHD studies should be able to better separate the direct effects of the drug on the immune system from the underlying disease and its prior therapies. The current resurgent interest in the basic biology of chronic GVHD and correlative studies done in these patients32,33 will hopefully allow this level of immune dissection in the future.
Although long-term outcome was not a primary outcome of the study, the 3-year projected overall survival of 60% is encouraging. There are very few publications reporting the long-term outcome of chronic GVHD in children34 and even less data about the long-term outcome of children with chronic GVHD needing salvage therapy. In a retrospective Italian Association for Pediatric Hematology and Oncology study, the 3-year disease-free survival of a large cohort of children newly diagnosed with chronic GVHD was approximately 70%.34 The 3-year survival of 60% in our study is therefore encouraging, given that all had failed initial conventional therapy. Likewise, the trend toward improved survival in patients responding to therapy (vs those not responding) with pentostatin is heartening. Therefore, one can view response as a potential surrogate marker for long-term survival. This observation is consistent with other studies in which complete or partial response of the chronic GVHD is associated with less non–relapse mortality and thus increased survival.7,35
In conclusion, we have shown, in a multi-institutional study, a response rate of 53% in pediatric patients treated with pentostatin for corticosteroid-refractory chronic GVHD. Toxicity was acceptable, and long-term survival is encouraging. We strongly support future multi-institutional studies to be performed through the PBMTC. We also recommend the study of pentostatin in patients with newly diagnosed, high-risk36 chronic GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all members (physicians and research nurses) of the Pediatric Blood and Marrow Transplant Consortium (PBMTC). The members are listed on the website www.pbmtc.org.
This work was supported by a grant from SuperGen Inc and Hospira Inc, and an investigator-initiated study (D.A.J.).
Authorship
Contribution: D.A.J. and G.B.V. wrote the protocol and the manuscript and analyzed data; A.L.G. contributed to the protocol design; A.R. contributed to the protocol design, provided statistical support, and wrote parts of the manuscript; B.B., M.G., L.L., E.R.N., and K.T. participated in data collection; and K.R.S. provided scientific support and helped analyze the data. All authors contributed to writing the paper and checked the final version.
Conflict-of-interest disclosure: D.A.J. has received honoraria as part of speakers' bureaus from SuperGen Inc and Hospira Inc. The remaining authors declare no competing financial interests.
A complete list of the members of the Pediatric Blood and Marrow Transplant Consortium appears in the online supplemental data.
Correspondence: David A. Jacobsohn, Northwestern University School of Medicine, Stem Cell Transplant Program, Children's Memorial Hospital, 2300 Children's Plaza, Box 30, Chicago, IL 60614; e-mail: djacobsohn@childrensmemorial.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal