Abstract
IMiDs immunomodulatory drugs, including lenalidomide and pomalidomide represent a novel class of small molecule anticancer and anti-inflammatory drugs with broad biologic activities. However, the molecular mechanism through which these drugs exert their effects is largely undefined. Using pomalidomide and primary human monocytes, we report that pomalidomide rapidly and selectively activated RhoA and Rac1, but not Cdc42 or Ras, in the absence of any costimulation. Consistent with the activation of Rho GTPases, we found that pomalidomide enhanced F-actin formation, stabilized microtubules, and increased cell migration, all of which were blocked by selective inhibitors of ROCK1 and Rac1. Further, we showed that in Swiss 3T3 cells, pomalidomide only activated RhoA, not Rac1 or Cdc42, and potently induced stress fiber formation. The pomalidomide effect on actin cytoskeleton was blocked by the ROCK1 inhibitor, but not Rac1 inhibitor. Finally, we demonstrated that pomalidomide was able to regulate the activity of Rho GTPases and the formation of F-actin in primary human T cells as it did in monocytes and showed that the activation of RhoA was essential for pomalidomide-induced interleukin-2 expression in T cells. These novel activities provide what we believe a critical mechanism by which IMiDs drugs function as therapeutic immunomodulatory agents.
Introduction
IMiDs immunomodulatory drugs were originally characterized for their ability to inhibit lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α) production in peripheral blood mononuclear cells (PBMCs) and to enhance interleukin-2 (IL-2) and interferon-γ (IFN-γ) expression in activated T cells.1,2 Broad biologic and pharmacologic activities have been demonstrated in a variety of in vitro and in vivo systems.3-5 One of the IMiDs drugs, lenalidomide, has been approved for the treatment of 5q− myelodysplasia (MDS) and for multiple myeloma (MM).6,7 Clinical studies on lenalidomide have also demonstrated its efficacy in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL).8-11 Pomalidomide, another potent IMiDs drug, is currently in clinical trials for several indications including MM, myelofibrosis, sickle cell anemia, and solid tumors.3 In vitro, immunomodulatory, proapoptotic, antiangiogenic, and antiproliferative activities have been demonstrated for IMiDs drugs in different cellular models.4,5,12,13 While each of these activities has been implicated to the anticancer activities observed clinically, a unifying molecular mechanism has not been demonstrated, if indeed one exists to explain the apparently diverse functional effects of these drugs. We have been particularly interested in IMiDs drug effects in the enhancement of T-cell and natural killer (NK) cell immune activity and the possibility that this is crucial for therapeutic response seen clinically in MM, CLL, and NHL patients. For example, IMiDs compounds were found to induce proliferation of T cells from healthy volunteers and MM patients in vitro after CD3 activation.2,14 Both lenalidomide and pomalidomide have been demonstrated to increase IL-2 and IFN-γ production in activated T cells, to activate NK cells, and enhance antibody-dependent cellular cytotoxicity (ADCC).15 Recently, Ramsay et al have shown that lenalidomide enhanced formation of the immunologic synapse between autologous T cells and CLL cells.16
To study the molecular mechanism of action of this class of drugs, we have focused on the proximal and immediate molecular events that follow exposure of cells to IMiDs drugs in the absence of any costimulation. From extensive gene expression profiling, pathway mapping, and second messenger (cAMP, cGMP) analysis in both activated monocytes and macrophages treated with IMiDs compounds, we obtained convergent evidence that linked IMiDs compounds with small GTPases (data not shown). We therefore decided to directly investigate the effect of pomalidomide on the cellular activity of Rho GTPases and their downstream cellular activities. Rho GTPases, comprising RhoA, Rac1, and Cdc42 as the most studied family members, are molecular switches that control a variety of fundamental biologic processes including cell differentiation, cell division, and cell movement.17,18 They are master regulators for both actin and microtubule cytoskeleton dynamics. In this report, we provide evidence from multiple cell models that IMiDs drugs have a profound effect on the cytoskeleton through selective activation of Rho GTPases. We further show that this novel activity of IMiDs drugs plays an important role in T activation. We propose that the modulation of GTPases and the cytoskeleton represents a fundamental molecular mechanism whereby IMiDs compounds change the interplay and environment of tumor cells and host immune cells.
Methods
Reagents
RPMI 1640 media and macrophage colony-stimulating factor (M-CSF) were from Invitrogen. Cell migration assay kit was from Thermo Scientific. Chemicals were purchased from Sigma-Aldrich unless specifically mentioned. Antibodies were from Cell Signaling if not specifically indicated. Pomalidomide and lenalidomide (Celgene Corporation) were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in all experiments was 0.003%. For most studies, 1 μM pomalidomide, a clinically relevant concentration, was used unless indicated otherwise.
Preparation of human monocytes and CD4+ T cells
Processed buffy coats (50 mL) were purchased from the San Diego Blood Bank. PBMCs were isolated by Ficoll gradient centrifugation as described.1 Monocytes or T cells were purified from PBMCs using anti-CD14 or anti-CD4 microbeads packed in Miltenyi MidiMACS columns (Miltenyi Biotec), respectively, according to the manufacturer's protocol.
RhoA, Rac1, Cdc42, and Ras activation assay in vitro
RhoA, Rac1, Cdc42, and Ras activities were measured by RhoA, Rac1, Cdc42, and Ras-GTP binding to their corresponding effector binding domains fused with glutathione S-transferase (GST) in pull-down assays following the manufacturer's protocol (Millipore). Briefly, the cell lysates were added to the Rhotekin (for RhoA), PAK-PBD (for Rac1 and Cdc42) or Raf-RBD (for Ras) beads, followed by washing 3 times with the washing buffer (Millipore). The GTP-bound GTPases associated with beads were analyzed by Western blot analysis. Activation of RhoA and Rac1 were also followed using G-LISA activation assay kits from Cytoskeleton according to the manufacturer's instructions with a minor modification. Cell extracts were added to 96-well plate coated with GST fusion of Rho binding domain. After incubation with light shaking at 4°C for 30 minutes, the plate was washed 3 times with washing buffer (Cytoskeleton) before addition of antigen presenting buffer (Cytoskeleton). The captured GTP-bound Rho GTPases were incubated with their corresponding anti-Rho GTPase antibodies. The GTPase-antibody conjugates were detected with horseradish peroxidase (HRP)–conjugated secondary antibody and quantified using the Victor 2, 1420 Multilabel Counter (PerkinElmer).
F-actin staining
F-actin staining was performed with a Rhodamine-Phalloidin–based kit (Cytoskeleton) according to the manufacturer's instructions with modifications. Freshly purified monocytes or T cells were plated on a 96-well plate. After treatments, cells were fixed on the plate by incubating with fixative solution for 10 minutes. Cells were permeabilized for 5 minutes and washed with 200 μL washing buffer (Cytoskeleton). Rhodamine-Phalloidin stock was loaded in and incubated for 30 minutes before addition of 50 μL cold phosphate-buffered saline (PBS). The cellular staining on actin filaments in monocytes and T cells was viewed by ArrayScan HCS Reader (Cellomics). Actin cytoskeleton in Swiss 3T3 cells was detected as described19 and analyzed with confocal microscopy using a Nikon Eclipse E800 (Nikon) equipped with a CoolSNAP HQ2 camera (Photometrics).
Microtubule assay
Microtubule content was determined using a microtubule stabilization kit (Cytoskeleton). Treated cells were homogenized in microtubule stabilization buffer (Cytoskeleton). Cell fraction containing microtubules was collected from cell extract by centrifugation at 100000g for 30 minutes. This microtubules-containing fraction was then incubated with 10 mM CaCl2 on ice for 1 hour. The resulted mixtures were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Tubulins were detected by a Western blot analysis.
Monocyte migration assays
Cell migration was quantified by Chemicon 5-μm 96-well Migration Assay kits (Chemicon, Millipore) according to the manufacturer's instructions with modifications. Plates and reagents were brought to room temperature before the initiation of assay. RPMI 1640 (150 μL) was loaded in the presence or absence of chemoattractant, M-CSF, to the wells of the bottom chamber. Cells (2 × 105) were placed in 100 μL into a migration chamber. Plates were covered and incubated for 3 hours at 37°C in a CO2 incubator (5% CO2). Cells were washed completely from the underside of the membrane and combined with cells that migrated into the medium in the lower chamber. Lysis buffer and dye solution (Chemicon, Milliopore) were added to the solution containing migratory cells, which was then transferred to a new 96-well plate and read with the Victor 2, 1420 Multilabel Counter.
IL-2 detection
Cell culture supernatant (25 μL) was used to quantify secreted IL-2 using an enzyme-linked immunosorbent assay (ELISA) from Mesoscale Discovery System according to the manufacturer's instructions. Briefly, detection antibody solution was incubated with cell supernatant at room temperature for 2 hours, followed by washing 3 times with PBS plus 0.05% Tween-20. IL-2 level was determined by a SECTORTM Imager Reader (Mesoscale Discovery System) in the presence of 150 μL 2× red buffer in each well.
Results
Pomalidomide selectively activates Rho family GTPases, RhoA, and Rac1, in human primary monocytes
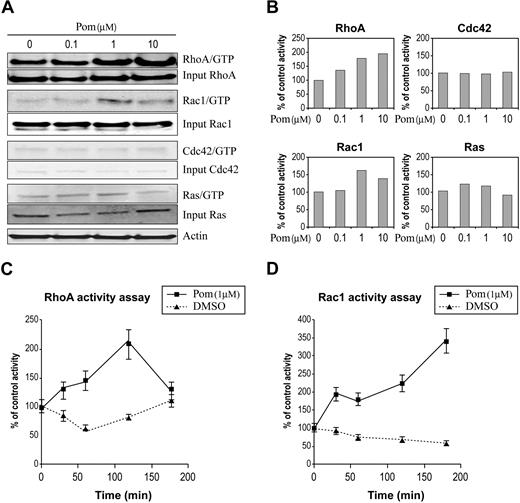
A complex protein network regulates GTPase cellular activity by switching them between GTP-bound active and GDP-bound inactive forms. The unique conformation of GTP-bound GTPases allows the activated GTPases to associate with downstream effectors that in turn carry out specific biologic responses. To examine the effect of pomalidomide on the cellular GTPases activity, we chose primary human monocytes isolated from peripheral blood of healthy donors as a cell model because of their robust response to IMiDs drugs and preliminary evidence that implicates IMiDs drugs with Rho GTPase activity signaling in these cells (data not shown). We treated the monocytes with pomalidomide and measured the amount of active GTPase. This was performed by capturing GTP-bound GTPase from cell lysates using immobilized GST-fusion proteins with domains from Rac1 and Cdc42 effector protein, p21 activated kinase 1 (PAK), and RhoA effector protein Rhotekin to detect the corresponding GTPases. A GST-fusion with Raf1 domain was used to bind active Ras. While pomalidomide treatment activated RhoA and Rac1, it showed little or no effect on either Cdc42 or Ras (Figure 1A-B). To examine the kinetics of the activation, we ran a time course where we saw acute temporal activation of RhoA and Rac1 (Figure 1C-D). No activation of Cdc42 was seen in the same assay. The activation of both RhoA and Rac1 was rapid upon pomalidomide treatment but showed different kinetics. This result represents one of the earliest cellular activities reported with IMiDs immunomodulatory drugs.
Pomalidomide selectively activates Rho GTPases in primary human monocytes. (A) RhoA, Rac1, Cdc42, and Ras activities were measured by pull-down assay in primary monocytes in the presence of pomalidomide at indicated concentrations for 30 minutes. (B) Quantitation of GTPases activation in panel A using densitometry is expressed as percent control relative to DMSO treatment. (C-D) RhoA and Rac1 activity were determined by G-LISA assay in the presence of 1 μM pomalidomide at indicated time points. These results are representative of 3 independent experiments.
Pomalidomide selectively activates Rho GTPases in primary human monocytes. (A) RhoA, Rac1, Cdc42, and Ras activities were measured by pull-down assay in primary monocytes in the presence of pomalidomide at indicated concentrations for 30 minutes. (B) Quantitation of GTPases activation in panel A using densitometry is expressed as percent control relative to DMSO treatment. (C-D) RhoA and Rac1 activity were determined by G-LISA assay in the presence of 1 μM pomalidomide at indicated time points. These results are representative of 3 independent experiments.
Pomalidomide induces cytoskeleton reorganization in monocytes
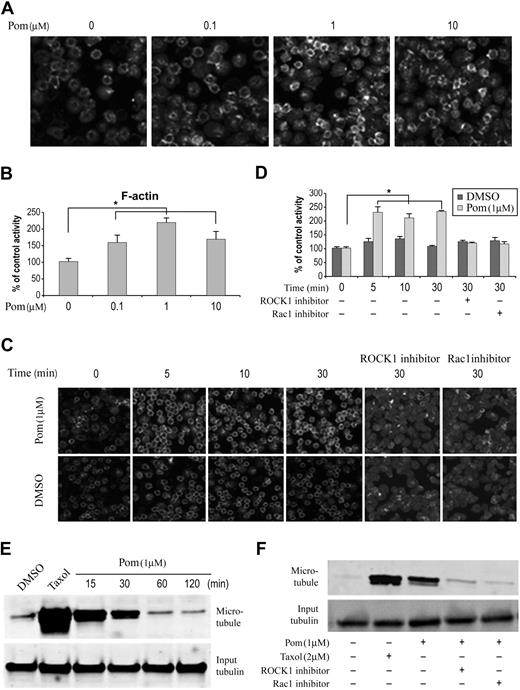
Cytoskeletal changes are among the best characterized and most profound cellular responses observed upon activation of Rho family GTPases.18,20 Through their downstream effector molecules, activated Rho GTPases regulate actin and microtubule cytoskeletal dynamics through polymerization, stabilization, and reorganization. With respect to the actin cytoskeleton, activation of each member of the Rho GTPase family shows a distinct effect with Rho, Rac, and Cdc42 leading to stress fiber, protrusive lamellipodia, and protrusive filopodia, respectively.20 To test if pomalidomide affected actin cytoskeleton, we treated monocytes with pomalidomide for 30 minutes and measured the F-actin content using fluorescent-phalloidin staining. We found that pomalidomide increased F-actin formation with optimal activity at a clinically relevant concentration of 1 μM. (Figure 2A-B). This pomalidomide-induced formation of F-actin occurred within 5 minutes of compound addition (Figure 2C-D). To confirm that pomalidomide activation of RhoA and Rac1 plays a role in this process, Y27632, a specific inhibitor of ROCK1,21 a RhoA downstream effector kinase that has been shown to mediate RhoA effect on actin cytoskeleton, and a Rac1-specific inhibitor19 were added to cells before pomalidomide treatment. Both inhibitors were able to block pomalidomide-induced F-actin formation (Figure 2C-D). However, due to the small size and shape of primary monocyte, it was difficult to define the specific type of actin filaments formed, particularly those induced by activation of RhoA. Unlike many adherent cells, monocytes do not possess stress fibers controlled by RhoA, rather activation of RhoA results in a rounded and contracted morphology that is technically difficult to discern. Therefore, we decided to use Swiss 3T3 cells to better define specific drug-induced changes in actin filament structure, which is a well-characterized cell model for studying actin cytoskeleton reorganization (data shown in Figure 4).
Pomalidomide regulates cytoskeleton in monocytes through Rho GTPases. (A-B) Monocytes were treated with pomalidomide at the indicated concentration or DMSO as a control for 30 minutes, followed by rhodamine-phalloidin staining. Quantitation of staining is normalized and expressed as percent control relative to DMSO treatment. Data represent 3 independent experiments. Columns and error bars represent mean ± SEM (Student t test, *P < .05). (C-D) Representative experiment shows ROCK1 and Rac1 inhibitors decrease pomalidomide-induced F-actin formation in monocytes. Monocyte cells were treated with 1 μM pomalidomide for indicated time points. When ROCK1 or Rac1 inhibitors were used, cells were preincubated for 30 minutes in the presence of 10 μM ROCK1 inhibitor (Y27632) or Rac1 inhibitor, followed by DMSO or pomalidomide incubation. Quantitation of staining (D) is normalized and expressed as percent control relative to DMSO treatment. Data represent 3 independent experiments. Columns and error bars represent mean ± SEM (Student t test, *P < .05). (E) Pomalidomide stabilizes microtubule in human primary monocytes. Cells were treated with DMSO or 1 μM pomalidomide for indicated time points. Microtubule level was determined by Western blot analysis using tubulin antibody after the microtubule depolymerization. Treatment with 2 μM Taxol for 2 hours was used as a positive control. (F) ROCK1 and Rac1 inhibitors block pomalidomide-induced microtubule stabilization. Monocytes were pretreated with ROCK1 or Rac1 inhibitors for 15 minutes before pomalidomide stimulation. Treatment with 2 μM Taxol for 2 hours was used as a positive control.
Pomalidomide regulates cytoskeleton in monocytes through Rho GTPases. (A-B) Monocytes were treated with pomalidomide at the indicated concentration or DMSO as a control for 30 minutes, followed by rhodamine-phalloidin staining. Quantitation of staining is normalized and expressed as percent control relative to DMSO treatment. Data represent 3 independent experiments. Columns and error bars represent mean ± SEM (Student t test, *P < .05). (C-D) Representative experiment shows ROCK1 and Rac1 inhibitors decrease pomalidomide-induced F-actin formation in monocytes. Monocyte cells were treated with 1 μM pomalidomide for indicated time points. When ROCK1 or Rac1 inhibitors were used, cells were preincubated for 30 minutes in the presence of 10 μM ROCK1 inhibitor (Y27632) or Rac1 inhibitor, followed by DMSO or pomalidomide incubation. Quantitation of staining (D) is normalized and expressed as percent control relative to DMSO treatment. Data represent 3 independent experiments. Columns and error bars represent mean ± SEM (Student t test, *P < .05). (E) Pomalidomide stabilizes microtubule in human primary monocytes. Cells were treated with DMSO or 1 μM pomalidomide for indicated time points. Microtubule level was determined by Western blot analysis using tubulin antibody after the microtubule depolymerization. Treatment with 2 μM Taxol for 2 hours was used as a positive control. (F) ROCK1 and Rac1 inhibitors block pomalidomide-induced microtubule stabilization. Monocytes were pretreated with ROCK1 or Rac1 inhibitors for 15 minutes before pomalidomide stimulation. Treatment with 2 μM Taxol for 2 hours was used as a positive control.
Besides their regulatory role on actin cytoskeleton, Rho family GTPases affect microtubule dynamics through stabilization.18 We then examined if activation of RhoA and Rac1 by pomalidomide had any effect on microtubules. As shown in Figure 2E, pomalidomide rapidly, but transiently, stabilized microtubules, albeit less robustly than taxol, which serves as a positive control. The microtubule stabilization induced by pomalidomide was inhibited by ROCK1 and Rac1 inhibitors, suggesting both GTPase activities are required (Figure 2F). This result supports an important role of Rho GTPases in mediating pomalidomide activity in microtubule dynamics. Thus, IMiDs drugs–mediated activation of Rho GTPases display the capacity to modulate 2 major components of cytoskeleton regulation, specifically actin and microtubule reorganization.
Pomalidomide enhances monocyte cell migration
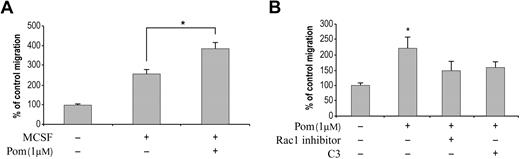
The role of Rho GTPases in promoting cell migration has been well established.18,22 The coordinated action for the formation of actin filament-dependent leading edge and contraction of actin-myosin at the trailing edge of the cell are required for cell migration. Through its downstream effector WAVE, a Wiskott-Aldrich syndrome protein, Rac1 promotes actin polymerization, which results in the formation of the leading edge at the front of the migrating cell.18,23 RhoA, through its effector ROCK1, is responsible for the generation of contractile force in the back of the cell by inducing the assembly and stabilization of actin:myosin filaments.18,24 To test whether activation of RhoA and Rac1 by pomalidomide would have an effect on cell migration, we used the Boyden chamber cell migration assay with M-CSF as chemoattractant. The addition of pomalidomide at 1 μM enhanced M-CSF–induced directional migration (Figure 3A) that occurred in a dose-dependent manner (data not shown). Significantly, Rho-specific inhibitor C3 and Rac1 inhibitor both blocked the migration induced by pomalidomide in the presence of M-CSF (Figure 3B). Similar inhibitory activity on cell migration by ROCK1 inhibitor was also observed, further supporting the role of RhoA in pomalidomide-induced cell migration (data not shown).
Pomalidomide induces monocyte migration. (A) Primary monocytes loaded on the upper chamber were incubated with DMSO or 1 μM pomalidomide. M-CSF was loaded on the bottom chamber as chemoattractant. Cells were allowed to migrate for 6 hours, and the migrated cells in the bottom chamber were determined. The amount of cells treated with DMSO is calculated as 100%. (B) Both Rho inhibitor C3 and Rac1 inhibitor decrease pomalidomide-induced monocyte migration. When C3 or Rac1 inhibitors were used, cells were preincubated with the inhibitors for 30 minutes before the addition of DMSO or pomalidomide. The amount of cells treated with DMSO is calculated as 100%. Columns and error bars represent mean (± SEM). Data represent average of 5 independent experiments (Student t test, *P < .05).
Pomalidomide induces monocyte migration. (A) Primary monocytes loaded on the upper chamber were incubated with DMSO or 1 μM pomalidomide. M-CSF was loaded on the bottom chamber as chemoattractant. Cells were allowed to migrate for 6 hours, and the migrated cells in the bottom chamber were determined. The amount of cells treated with DMSO is calculated as 100%. (B) Both Rho inhibitor C3 and Rac1 inhibitor decrease pomalidomide-induced monocyte migration. When C3 or Rac1 inhibitors were used, cells were preincubated with the inhibitors for 30 minutes before the addition of DMSO or pomalidomide. The amount of cells treated with DMSO is calculated as 100%. Columns and error bars represent mean (± SEM). Data represent average of 5 independent experiments (Student t test, *P < .05).
Pomalidomide induces stress fiber formation in Swiss 3T3 cells through activation of RhoA
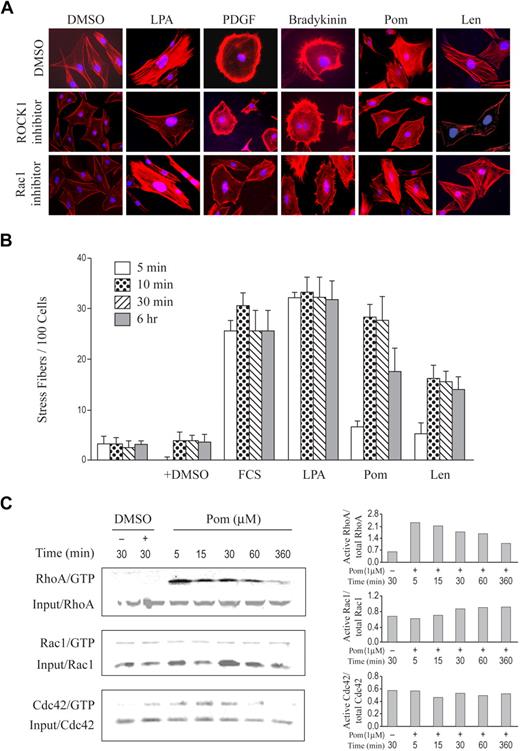
While complex and probably context-specific, we reasoned that the fundamentals of Rho GTPase regulation of cytoskeleton dynamics in human monocytes might be conserved in other cell types and in other species. Most studies with IMiDs compounds have been performed using human cellular models, and there is some evidence that effects seen in human cells have translated poorly in rodent cells. We hoped that examination of pomalidomide effects in rodent cells might highlight differences in how specific Rho GTPases are regulated by IMiDs class of drugs and how this corresponds to species-specific actin cytoskeleton changes. We chose Swiss 3T3 murine cells, one of the best-characterized cell models for studying cytoskeletal change, because the specific activator for each of the GTPases, RhoA, Rac1, Cdc42, and the corresponding cytoskeletal phenotype has been well investigated.20 As reported and established in the literature,20 treatment of Swiss 3T3 cells with lysophosphatidic acid (LPA), platelet-derived growth factor (PDGF), or bradykinin-activated RhoA, Rac1, and Cdc42, respectively, which in turn induced the formation of distinct actin filaments that were either stress fibers, lamellipodia, or filopodia, respectively (Figure 4A). Treatment with pomalidomide induced stress fibers that appeared almost identical to what was formed after treatment with LPA (Figure 4A). ROCK1 inhibitor, but not Rac1 inhibitor, abolished both pomalidomide and LPA effects, suggesting that activation of RhoA is required. As expected, Rac1 inhibitor was able to block PDGF-induced lamellipodia (Figure 4A). Like pomalidomide, lenalidomide also induced stress fiber formation (Figure 4A). To further characterize pomalidomide and lenalidomide effects on stress fiber formation, we performed a time course measuring IMiDs effect in comparison with serum or LPA positive controls. As shown in Figure 4B, 1 μM pomalidomide was as robust an inducer of stress fiber formation as LPA or serum. Lenalidomide also induced similar actin filaments, but was less potent, consistent with other assays that have shown lenalidomide being less active than pomalidomide (Figure 4A-B).4,5 Interestingly, both serum and LPA showed faster onset than pomalidomide or lenalidomide, which may reflect the difference of their mode of action. To determine whether pomalidomide can indeed affect RhoA cellular activity in these cells, we examined the activation status of RhoA, Rac1, and Cdc42 after pomalidomide treatment. We found that pomalidomide strongly activated RhoA as early as in 5 minutes, while it had little or no effect on either Rac1 or Cdc42 (Figure 4C). Notably, activation of RhoA by pomalidomide occurs before the induction of stress fibers (Figure 4B-C). This result confirms the ability of pomalidomide to activate RhoA and shows that activation of Rho GTPases by pomalidomide is context-dependent. We have also found that pomalidomide failed to activate any of 3 members of Rho GTPases in some tumor lines such as Namalwa (data not shown). Therefore context-dependent and selective activation of Rho GTPases and regulation of Rho-controlled cellular activities by IMiDs drugs may have important mechanistic and therapeutic implications that deserve further investigation.
Pomalidomide and lenalidomide induce stress fiber formation in Swiss 3T3 cells through activation of RhoA. (A) Swiss 3T3 fibroblasts were incubated in serum-free medium with 0.2% NaHCO3 for 20 hours before stimulation with 0.01% DMSO, 20 ng/mL LPA, 3 ng/mL PDGF, 100 ng/mL bradykinin, 1 μM pomalidomide, or 1 μM lenalidomide for 30 minutes. Where indicated, 30 μM ROCK1 inhibitor or 30 μM Rac1 inhibitor were added to cells 12 hours before the above stimulations. The cells were then fixed with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained with Alexa 594 phalloidin to visualize F-actin (red). (B) Serum-starved cells were stimulated as indicated in a time course and were followed by Alex-594 phalloidin staining. Each bar represents the percentage of cells with stress fiber clearly observed under each condition, which were quantified in randomly chosen 100 cells per a 22 × 22-mm glass coverslip. Results shown here are the mean of 3 independent experiments. Error bars indicate ± SE. (C) Serum-starved cells were treated with 1 μM pomalidomide for the indicated time. Rho GTPases activity was analyzed by the GTPase pull-down assay as described in “Methods.” Quantification for each treatment condition is shown on the right panel.
Pomalidomide and lenalidomide induce stress fiber formation in Swiss 3T3 cells through activation of RhoA. (A) Swiss 3T3 fibroblasts were incubated in serum-free medium with 0.2% NaHCO3 for 20 hours before stimulation with 0.01% DMSO, 20 ng/mL LPA, 3 ng/mL PDGF, 100 ng/mL bradykinin, 1 μM pomalidomide, or 1 μM lenalidomide for 30 minutes. Where indicated, 30 μM ROCK1 inhibitor or 30 μM Rac1 inhibitor were added to cells 12 hours before the above stimulations. The cells were then fixed with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained with Alexa 594 phalloidin to visualize F-actin (red). (B) Serum-starved cells were stimulated as indicated in a time course and were followed by Alex-594 phalloidin staining. Each bar represents the percentage of cells with stress fiber clearly observed under each condition, which were quantified in randomly chosen 100 cells per a 22 × 22-mm glass coverslip. Results shown here are the mean of 3 independent experiments. Error bars indicate ± SE. (C) Serum-starved cells were treated with 1 μM pomalidomide for the indicated time. Rho GTPases activity was analyzed by the GTPase pull-down assay as described in “Methods.” Quantification for each treatment condition is shown on the right panel.
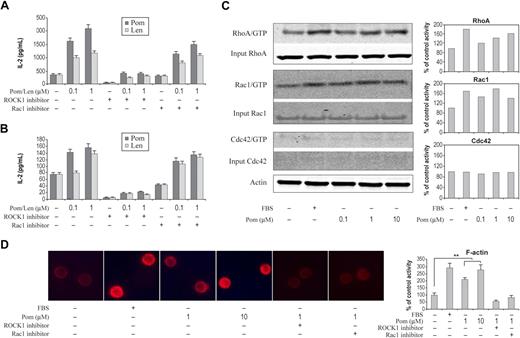
IMiDs drugs activate Rho GTPases, induce F-actin formation, and enhance IL-2 gene expression in primary T cells via RhoA
IL-2 is an important cytokine for T-cell proliferation and activation of T cells and NK cells. Induction of IL-2 gene expression in T cell is considered to be a pivotal step in T-cell activation. Optimal T-cell proliferation and activation requires signaling from both T-cell receptor (TCR) and CD28 costimulation.25 Biochemical and biophysical analysis of costimulation of TCR and CD28 revealed that the integration of these signals occurred proximal to the cell membrane but not the nucleus.25 Rho family GTPases, RhoA, Rac1, Cdc42, and their guanine nucleotide exchange factor Vav1 have been reported to be key converging points for T-cell activation and IL-2 expression following costimulation by TCR and CD28.25,26 IMiDs class of drugs have been shown to activate T cells and to stimulate T-cell cytotoxic activity through CD28 pathway and by inducing cytokines such as IL-2.14,15,27,28 The induction of IL-2 by IMiDs drugs was demonstrated in T cells coactivated by either CD3 ligation or treatment with phorbol myristate acetate (PMA).17,29 To test the involvement of Rho GTPases in IMiDs drugs–induced IL-2 expression in activated T cells, we treated primary CD4+ T cells activated by anti-CD3 with pomalidomide or lenalidomide in the presence or absence of specific ROCK1 and Rac1 inhibitors and then followed IL-2 protein expression by ELISA. As shown in Figure 5A, both pomalidomide and lenalidomide potentiated CD3-induced IL-2 expression, which is sensitive to the inhibition of ROCK1 inhibitor, but not Rac1 inhibitor. Furthermore, both pomalidomide and lenalidomide were able to stimulate IL-2 expression without costimulation (Figure 5B). ROCK1 inhibitor abolished such IL-2 expression by both IMiDs drugs, while Rac1 inhibitor showed minimum effect (Figure 5B). This result supports the important role of RhoA in mediating IMiDs drug response and unites an established IMiDs functional effect within the GTPase hypothesis. This activity in T cells was confirmed directly by examining the effect on Rho GTPases after pomalidomide treatment. We found that both RhoA and Rac1 were activated by pomalidomide (Figure 5C) and that pomalidomide-induced F-actin formation in T cells was dependent on RhoA and Rac1 activity (Figure 5D).
Activation of Rho GTPases, induction of IL-2, and formation of F-actin by IMiDs. (A) CD4+ T cells were treated with pomalidomide or lenalidomide for 6 hours at the indicated concentration in the presence of anti-CD3 antibody (A) or without anti-CD3 antibody (B). Cells were preincubated for 30 minutes with ROCK1 inhibitor or Rac1 inhibitor followed by pomalidomide or lenalidomide incubation. IL-2 level was measured by Mesoscale Discovery System. Columns and error bars represent mean (± SEM). (C) Pomalidomide selectively activates RhoA, Rac1. Small GTPase activities in CD4+ cells were measured by pull-down assay in the presence of pomalidomide at indicated concentrations for 30 minutes. Quantitation of pull-down protein is normalized to actin and expressed as percent control relative to DMSO treatment. (D) Representative experiment shows that the staining of F-actin by rhodamine-phalloidin, from nonstimulated (DMSO) or stimulated (pomalidomide) CD4+ T cells for indicated concentrations in the presence or absence of ROCK1 and Rac1 inhibitors. Cells were preincubated for 30 minutes in the presence of 10 μM ROCK1 inhibitor or Rac1 inhibitor followed by DMSO or pomalidomide stimulation. F-actin staining of 300 cells from 3 random fields was quantified in each experiment condition (right panel). Data represent 3 independent experiments. Columns and error bars represent mean ± SEM (Student t test, *P < .01).
Activation of Rho GTPases, induction of IL-2, and formation of F-actin by IMiDs. (A) CD4+ T cells were treated with pomalidomide or lenalidomide for 6 hours at the indicated concentration in the presence of anti-CD3 antibody (A) or without anti-CD3 antibody (B). Cells were preincubated for 30 minutes with ROCK1 inhibitor or Rac1 inhibitor followed by pomalidomide or lenalidomide incubation. IL-2 level was measured by Mesoscale Discovery System. Columns and error bars represent mean (± SEM). (C) Pomalidomide selectively activates RhoA, Rac1. Small GTPase activities in CD4+ cells were measured by pull-down assay in the presence of pomalidomide at indicated concentrations for 30 minutes. Quantitation of pull-down protein is normalized to actin and expressed as percent control relative to DMSO treatment. (D) Representative experiment shows that the staining of F-actin by rhodamine-phalloidin, from nonstimulated (DMSO) or stimulated (pomalidomide) CD4+ T cells for indicated concentrations in the presence or absence of ROCK1 and Rac1 inhibitors. Cells were preincubated for 30 minutes in the presence of 10 μM ROCK1 inhibitor or Rac1 inhibitor followed by DMSO or pomalidomide stimulation. F-actin staining of 300 cells from 3 random fields was quantified in each experiment condition (right panel). Data represent 3 independent experiments. Columns and error bars represent mean ± SEM (Student t test, *P < .01).
Discussion
The clinical benefit of lenalidomide in treating severe hematologic disorders including MDS, MM, CLL, and NHL, has drawn heightened attention to how this class of drugs works at the molecular level, since the specific binding targets of IMiDs drugs remain unknown. Pomalidomide, a structurally related and more potent IMiDs drug than lenalidomide, was used for most of the studies in this report to investigate mechanism of action of this class of drugs.
We report for the first time that IMiDs immunomodulatory drugs regulate cytoskeleton reorganization through selective activation of Rho family GTPases. To demonstrate this novel function of IMiDs drugs, we provided experimental evidence from multiple cell models including human primary monocytes and T cells and murine Swiss 3T3 cells. We show that pomalidomide activates RhoA in all 3 cell types. While pomalidomide also activates Rac1 in human monocytes and T cells, it fails to do so in murine Swiss 3T3 cells even though there is abundant Rac1 protein in those cells. We have yet to detect an effect on Cdc42 from cells treated with pomalidomide. In T cells, however, there was little total Cdc42 protein detected, so we do not know IMiDs drugs effect on Cdc42 activity. These results highlight that IMiDs drugs function in a context-dependent manner when modulating GTPase activity, which may be essential to provide a therapeutic window for this class of drugs, as activation of Rho GTPases can result in broad biologic effects. Because Rho family GTPases are often required to translocate to the cell membrane to carry our their functions, measurement of endogenous Rho GTPases cellular location and their association with downstream effector proteins upon IMiDs drug treatment in a well-defined cellular system would be interesting and should be further explored. The activity of Rho GTPases is tightly controlled by a complex protein network, comprising Rho-GTP exchange factors (GEFs), GDP dissociation inhibitors (GDIs), and GTPase activating proteins (GAPs).21 In addition, Rho GTPases have been shown to regulate each others' activity. At present, we do not fully understand how IMiDs drugs activate Rho GTPases. It is possible that IMiDs drugs bind to and modulate the function of any of these complexes. The rapid and selective activation of these GTPases by IMiDs drugs in different cell models described here should provide a powerful system for comparative investigation of the drug molecular target(s). For example, activation of RhoA in Swiss 3T3 cell should provide an excellent model to investigate if a GEF or GAP, known to be specific for RhoA, is involved in mediating pomalidomide effect.
Consistent with activation of Rho GTPases, pomalidomide causes dramatic actin and microtubule cytoskeleton changes that are dependent on Rho GTPase activation (Figures 2, 4, and 5). The activation of Rho GTPase by IMiDs drugs occurs before their effect on actin cytoskeleton reorganization (Figure 4). In both T cells and monocytes, the F-actin increase by pomalidomide can be inhibited by either ROCK1 or Rac1 inhibitor, suggesting that both RhoA and Rac1 activities are required for pomalidomide effect on F-actin. In fact, both RhoA and Rac1 are activated by pomalidomide in those cells (Figures 1 and 5). In Swiss 3T3 cells, pomalidomide only activates RhoA and induces the stress fiber formation that is sensitive only to RhoA inhibition as expected, but not to the inhibition of Rac1 (Figure 4). Because coordinated actions from both actin and microtubule cytoskeleton are necessary for directed cell migration, we reasoned that pomalidomide should affect cell migration. We showed that pomalidomide is able to stimulate cell migration in monocytes that requires both RhoA and Rac1 activities (Figure 3). Significantly, we show that activation of Rho GTPases and cytoskeleton reorganization by pomalidomide is achieved in minutes after the cells are in contact with the drug, absent any costimulation. This provides important mechanistic insight for these drugs, since most reported studies of IMiDs drugs usually require costimulation and compound treatment for hours or days. The IMiDs immunomodulatory drugs function we describe here appears primary and proximal to published activities from these drugs and should provide an approach to search for the precise target(s).
Impaired and dysfunctional NK and T cells from tumor immunoediting have been well documented in cancer patients including MM and CLL.16,30-34 Therefore, reactivation of anticancer immunity of T cells and NK cells should benefit cancer patients. IMiDs have been shown to augment both NK and T-cell tumor cell lysis activity in MM and CLL patients.35-37 The observed immune enhancement from these drugs in vitro and in vivo was largely believed to be due to their ability to enhance IL-2 expression from activated T cells.3,14,38 In this report, we have demonstrated that IMiDs drugs activate RhoA in T cells and have shown that RhoA plays a critical role in lenalidomide- and pomilidomide-enhanced IL-2 expression costimulated by CD3 as well as IL-2 expression induced by these drugs alone (Figure 5). This is consistent with previous reports that show RhoA plays a regulatory role in IL-2 expression in T cells activated by either TCR or TCR and CD28 costimulation.39-41 In addition to the increased IL-2 expression through Rho GTPases, IMiDs drugs may enhance T cell and NK cell tumor killing activity through another aspect of Rho GTPases activation, the cytoskeleton reorganization. It has been well documented that Rho GTPases and actin cytoskeleton regulate multiple aspects of NK and T-cell development and activation.25,26,42 Through the formation of immunologic synapses, which are regulated by Rho GTPases and actin cytoskeleton reorganization, cytotoxic T cell and NK cells exert their tumor killing activity.42,43 We show that pomalidomide is able to activate Rho GTPases and increase F-actin in primary T cells just like it does in monocytes (Figure 5). Recently, Ramsay et al importantly showed that the CD4+ and CD8+ T cells from CLL patients failed to form immunologic synapses with autologous CLL blast cells.16 This defect in T cells was in part due to the impaired actin cytoskeleton. Further, the authors demonstrated that lenalidomide treatment of autologous T cells and CLL cells increased the amount of F-actin at the immune synapses and improved synapse formation.16 Our results described in this report are not only in agreement with their findings, but potentially provide a molecular mechanism that explains how IMiDs drugs enhance immune synapse formation. We have preliminary data to suggest that activation of Rho GTPases by IMiDs drugs is required for the improved immunologic synapse (data not shown). We hypothesize that the rapid and sustained activation of T cells and NK cells by IMiDs drugs through activation Rho GTPases and cytoskeleton reorganization is a key mechanism of drug-induced immune enhancement. This mechanism may also explain the clinical observation in many patients who lenalidomide seems to be effective after the first dose.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sophie Peng for technical support on microscopic analysis, Nathan Eller for assistance in figure preparation, and Sarah Cox and Pilgrim Jackson for comments on the manuscript. The authors thank George Muller for many insights on IMiDs drugs.
This work was supported by Celgene.
Authorship
Contribution: Y.X. and J.L. performed the majority of the experiments and did data analysis; G.D.F. and D.K. performedexperiment; A.L.G., L.G.C., and D.R.W. provided reagents; F.M. and B.L.B. provided the early thesis, research advice, and interpreted the data; and W.X. provided early thesis, designed and directed the research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: All authors except F.M., a former Celgene employee, are currently employed by Celgene.
Correspondence: Weilin Xie, Celgene, 4550 Towne Centre Court, San Diego, CA 92121; e-mail: wxie@celgene.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal