Abstract
Receptors for the fragment crystallizable region of immunoglobulin-G (FcγRs) play an important role in linking the humoral and cellular arms of the immune response. In this study, we present a comprehensive functional comparison of 2 human Fc-receptors, FcγRI and FcγRIIa. Activation of FcγRI results in a novel signaling cascade that links phospholipase D1 to sphingosine kinase-1 in U937 cells and primary human monocytes. This induces the expression of proinflammatory mediators and is associated with trafficking of immune complexes into human leukocyte antigen-DM positive antigen-processing compartments coupled with improved MHC class II–mediated antigen presentation to T lymphocytes. In contrast, activation of FcγRIIa elicits signaling through phospholipase Cγ1, resulting in increases in intracellular calcium, activation of nicotinamide adenine dinucleotide phosphate-oxidative burst, and differential membrane trafficking combined with impaired antigen presentation and proinflammatory cytokine expression. These data provide a mechanistic insight into the disparate activities associated with Fc receptors in immunity, namely, reinforcement of immune responses through stimulation of proinflammatory signaling and antigen presentation, versus the maintenance of immunologic homeostasis through the noninflammatory clearance of immune complexes.
Introduction
Fragment crystallizable receptors (FcRs) are receptors on immune cells that bind to the Fc region of immunoglobulins. FcγRs that bind to the most common type of immunoglobulin (IgG), are expressed on the surface of many different immune cell types including monocytes, macrophages, dendritic cells, and neutrophils.1-3 In humans, 3 different classes of activatory IgG receptors have been defined: FcγRI (CD64), FcγRIIa (CD32a), and FcγRIII (CD16), each of which has a variety of isoforms with differing affinities for IgG, tissue distribution, and level of expression.1-6 The high affinity IgG receptor, FcγRI, is a 72-kD type-I membrane glycoprotein constitutively expressed on monocyte and macrophage lineage cells.4 FcγRI is a member of the multichain immune recognition receptor family, comprising hetero-oligomeric complexes of a ligand-binding α-chain and a signaling γ-chain usually found in association with other immune receptors.1-6 The γ-chain contains a signaling motif termed the “immunoreceptor tyrosine-based activation motif” (ITAM): it is through the ITAM-bearing chain that most FcRs trigger intracellular signal transduction cascades. The low-affinity receptor, FcγRIIa, is the most broadly distributed human FcγR and is expressed on many cell types, such as monocytes, neutrophils, and platelets.1,7 This low-affinity receptor preferentially binds complexes of IgG and is the only Fc receptor that contains an ITAM of its own. Thus, it is the only Fc receptor that does not need to oligomerize with a γ-chain in order to signal.4,8,9 There is no identified murine equivalent of FcγRIIa.1
On myeloid cells, aggregation of FcγRs during the early stages of infection leads to several cellular responses, including the internalization of immune complexes by endocytosis or opsonized particles through phagocytosis, degranulation with the release of proteases, activation of respiratory burst, and secretion of cytokines.5,10-12 The presentation of antigens derived from internalized complexes forms an important component of our adaptive immune response, and dysregulation of this pathway is reported to be linked to increased susceptibility to bacterial sepsis.13 The safe clearance of immune complexes toward the latter stages of infection is also dependent on FcR expressing mononuclear phagocytes. Dysfunction in the clearance of immune complexes is reported to be associated with immunopathology, autoimmunity, and allergic disease.9,14 This represents one of the critical but poorly understood functions of Fc receptors, ie, the determination of the antigenic fate of immune complexes; specifically, whether to internalize and digest them in a way that is noninflammatory or to reinforce antigen presentation combined with immune activation and associated proinflammatory signaling.
Studies on differential functions mediated by individual Fc receptors in immune activation/homeostasis are complicated by the coexistence of several FcRs on phagocytic cells—it is difficult to identify the signaling cascades and functions triggered by one specific receptor, when they are cross-linked by a multivalent ligand (ie, IgG complexes). Nevertheless, it has been shown that aggregation of FcγRI or FcγRIIa results in signal transduction events evidenced by tyrosine phosphorylation of proteins,2,12 calcium release from internal stores,8,15,16 and the activation of various phospholipases and lipid kinases.8,10,12,16-19 It has been intimated that FcγRI is constitutively associated with detergent-insoluble lipid microdomains in the absence of ligand cross-linking20 and that FcγRI−/− mice exhibit impaired antigen presentation and cytokine production.21 In contrast, FcγRII requires ligand cross-linking for association with lipid rafts.22 In this study, we compare and contrast the signaling pathways elicited by human FcγRI and FcγRIIa in U937 and primary human monocytes and address the role of these receptors in membrane trafficking and presentation of influenza A virus antigens delivered as part of human IgG immune complexes.
Methods
All the chemicals and reagents are of molecular biology grade and from Sigma-Aldrich unless otherwise specified. This research was conducted under a protocol approved by the National University of Singapore Institutional Review Board covering all work described with human blood-derived cells and antibodies. All blood was collected after informed consent was obtained in accordance with the Declaration of Helsinki.
Tissue culture and FcγRI and FcγRIIa receptor aggregation.
The human monocytic cell line U937 (ATCC) was grown in normal growth medium and treated with 100-ng/mL IFN-γ (PeproTech) overnight.23 The next day, the cells were washed and divided into 2 and treated with 1 μM of mouse anti–human CD64 and mouse anti–human CD32 antibodies (Murine IgG1 clones 10.1 and 3D3, respectively, BD Pharmingen) for 45 minutes at 4°C, with tumbling to occupy surface FcγRs. Murine IgG1 antibodies were used to ensure minimal cross-reactivity with human FcRs.24 Excess unbound ligand was removed by serial washing in medium. Cells were resuspended in ice-cold medium, and surface immune complexes were formed by incubating with cross-linking antibodies (goat anti–mouse IgG F(ab) ′2 1:50) for 10 minutes at 4°C. Cells were washed 2 times to remove unbound goat IgGs. Cells were then incubated at 37°C for the times stated in the Results section.
Preparation of primary human monocytes
Primary human monocytes were prepared from fresh human blood samples taken under informed consent from volunteer human donors. Monocytes in unfractionated peripheral blood exhibited significant surface staining with goat anti–mouse F(ab′)2 (Thermo Scientific), suggesting a significant degree of FcR occupancy by monomeric IgGs (see supplemental Figure 1Ai, available on the Blood website; see the Supplemental Materials link at the top of the online article). Purified monocytes were isolated using anti-CD14 microbeads (Miltenyi Biotec) and washed 8 times in 100× volumes of ice-cold citrate buffer (PBS/0.4% sodium citrate) before culture and use in serum-free medium (AIM V or DMEM, Invitrogen). Monocytes prepared in this way exhibit a significant reduction in surface staining for human IgG (see supplemental Figure 1Aii) and show significant binding activity for exogenous human IgG (see supplemental Figure 1Aiii). All monocytes used in this study were selected based on negligible surface staining for CD16 by flow cytometry (see supplemental Figure 2C).
Intracellular signaling assays in U937 and primary human monocytes
Cytosolic Ca2+ measurement; phospholipase C and phospholipase D activity, chemical inhibition experiments, fluorescent microscopy, and protein kinase C (PKC) enzyme activity assay; subcellular fractionation; and Western blots were performed as described previously.8,10,16-18,25-28 Antisense knockdown, antisense oligonucleotides were from 1st Base for PLD1, 5′-CCGTGGCTCGTTTTTCAGTG-3′; PLD1-scrambled control, 5′-TTCCTTGGGTTCCGCTTGGA-3′; PLCγ1, 5-GGGGACGCGGCGCCCGCCAT-3; and PLCγ1-scrambled control, 5-CTGGTGGAAGAAGAGGACGT-3. Transfections were performed as previously described.27-29 Phosphoprotein arrays, 8-plex phosphoprotein arrays (Bio-Rad) were used to assay cell lysates for phosphorylation of Akt, MEK, ERK1 or ERK2, p38, Jnk, NFκB, c-Jun, and Src. The assay was conducted per the manufacturer's instructions.
Effector response assays
For the nicotinamide adenine dinucleotide phosphate (NADPH)–oxidase assay, superoxide production was measured using an enhanced luminol-based substrate (National Diagnostics), as previously described.25 For the degranulation assay, degranulation was measured using a colorimetric assay to assess the release of β-hexosaminidase as previously described.27,28 For quantitative RT-PCR, quantitative PCR was performed as previously described.30,31 For amplicon detection, the LightCycler RNA Master SYBR Green Kit (Roche) was used as described by the manufacturer, and the PCR was performed in a LightCycler instrument (Roche). For the cytokine array, a 17-plex cytokine array (Bio-Rad) was used to assay the supernatants for the following targets: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, GM-CSF, IFN-γ, TNF-α, G-CSF, MCP-1, and MIP-1β. The assay was conducted per the manufacturer's instructions.
Stimulation of primary monocytes with immune complexes
The generation of human IgG/influenza A immune complexes is detailed in the supplementary Methods section. Monocytes were resuspended in 200 μL of serum-free AIM-V media (Invitrogen). To block FcγRI, 10 μm of human IgG (Sigma-Aldrich) was added to the cells. We confirmed that at this concentration, the immune complexes (IC) used for 10 minutes at a titration of 1:5 do not exhibit significant surface binding to FcγRI through displacement of surface monomeric IgG (see supplemental Figure 1B). To block FcγRII, 100 μg/mL of IV.3 monoclonal antibody was added to the cells (ATCC).32 The cells were incubated with these blocking antibodies for 1 hour at 4°C. They were then washed with PBS and resuspended in 200 μL of AIM-V media each; 25 μ L of immune complex was then added to each sample and incubated at 37°C for 10 minutes to induce immune complex internalization or cellular signaling.
T-cell proliferation assay
The generation of influenza-specific CD4+ T cells is detailed in the supplementary Materials section. α-PR8 CD4+ T cells were plated in a 96-well plate (100 μL of 2 × 106 cells/mL); 4 × 104 treated CD14+ were treated in the following way: (1) untreated, (2) FcγRI-induced immune complex uptake for 10 minutes, (3) FcγRII-induced immune complex uptake for 10 minutes, and (4) immune complex uptake for 10 minutes through both FcγRI and FcγRII. Aliquots of (1)-(4) were then added to the plate. The APC to T-cell ratio used was 1:5, and they were incubated at 37°C and 5% CO2 for 4 to 7 days, until the T cells could be seen to be proliferating. Cell proliferation was measured by the 3H-thymidine method as previously described.33
IFN-γ ELISPOT
The assay was carried out as previously described.34 Briefly, freshly isolated human peripheral mononuclear cells, washed 8 times in 100× volumes of ice-cold citrate buffer and depleted of CD8+ were treated with immune complexes as described above in steps 1 through 4 in “T-cell proliferation assay” and resuspended in AIM-V media (Invitrogen) with 2% human AB serum at a concentration of 2 × 106 cells/mL, then the cells were plated (100μL cells/well) into a 96-well plate. The plate was incubated at 37°C for 16-18 hours, washed, and 100 μL of 0.5 μg/mL biotinylated 7-B6-1 was added to each well and incubated for 2 hours; 100 μL of 1:2000 dilution of streptavidin-ALP was then added to each well and incubated for 1 hour; 50 μL of the BCIP/NBT substrate was then added to each well for 2 minutes. After a final wash the plate was left to dry and read using the CTL ImmunoSpot S4 Analyzer (Cellular Technology Ltd).
Statistical analysis
Results for the phosphoprotein array, IFN-γ ELISPOT, and the proliferation assay were first analyzed using analysis of variance (ANOVA). Upon obtaining a significant F value, a Tukey protected t test was then conducted, comparing the results obtained by stimulating the cells with either FcγRI and/or FcγRII cross-linking.
Results
Monocyte-differentiated U937 cells express FcγRI and FcγRIIa but not FcγRIIb1/2
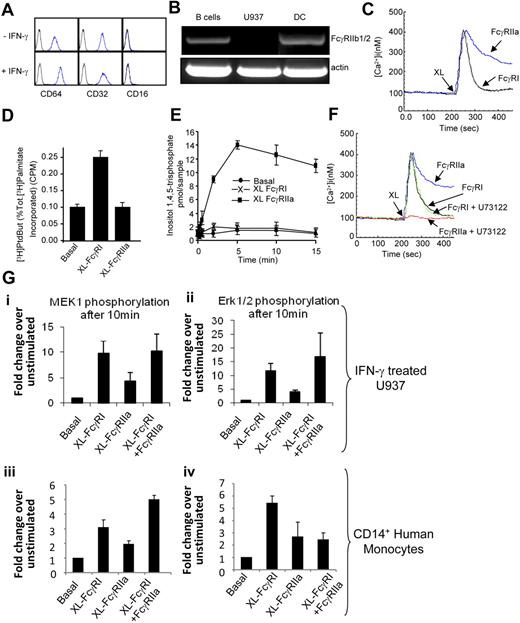
U937 is a premonocytic cell line that expresses high levels of surface FcγRIIa and low levels of surface FcγRI. When U937 cells are treated with IFN-γ, the expression of FcγRI detected on the cell surface by flow cytometry increases. U937 cells do not express FcγRIII under these culture conditions (Figure 1A). We performed RT-PCR analyses to test for expression of the immune-tyrosine inhibitory motif-containing inhibitory FcR, FcγRIIb1/2. Primers specific for FcγRIIb1/2 were used to detect the expression of this receptor in human B cells and dendritic cells.35 FcγRIIb1/2 transcripts were undetectable in U937 (Figure 1B).
FcR expression and signaling in U937 and primary monocytes. (A) U937 cells express FcγRI and FcγRII, but not FcγRIII, as determined by flow cytometry using anti-CD32PE and anti-CD64PE and anti-CD16PE (BD Pharmingen). (B) RT-PCR was used to assess the expression of FcγRIIb1/2 on U937 compared with B lymphocytes and dendritic cells. (C) FcγRI and FcγRIIa trigger differential cytosolic Ca2+ signals. Cytosolic calcium was measured in U937 by cuvette fluorimetry over 8 minutes after cross-linking of the individual FcRs using antibody clones 10.1 and 3D3 (BD Pharmingen), respectively (XL). (D) FcγRI triggers PLD activity, whereas FcγRIIa does not. PLD activity measured in resting U937 cells (Basal) or in cells after FcγR aggregation (XL FcγRI or XL FcγRIIa) for 30 minutes. (E) FcγRIIa triggers PLC activation in U937 cells. InsP3 generation was measured in resting cells (basal) or in cells after FcγR aggregation (XL FcγRI or XL FcγRIIa) for 15 minutes. (F) The PLC inhibitor U73122 blocks the FcγRIIa-mediated cytosolic calcium signal in U937 cells. Cells pretreated with the PLC inhibitor U73122 for 45 minutes were assayed for cytosolic calcium over 8 minutes after FcR cross-linking (XL FcγRI or XL FcγRIIa). (G) In IFN-γ–treated U937 (i-ii) and primary human monocytes (iii-iv), MEK1 and ERK1/2 are activated by FcγRI ligation by antibody clone 10.1 to a greater extent than by FcγRIIa ligation by antibody clone 3D3. Phosphoprotein array (Biorad) was used to measure ERK1/2 and MEK1 phosphorylation in cell lysates. MEK1, ERK, PLC, and PLD activity is expressed as a mean ± SD from 3 independent experiments. All intracellular calcium measurements were carried out in the presence of 1.5 M extracellular calcium and results shown are typical of 3 independent experiments.
FcR expression and signaling in U937 and primary monocytes. (A) U937 cells express FcγRI and FcγRII, but not FcγRIII, as determined by flow cytometry using anti-CD32PE and anti-CD64PE and anti-CD16PE (BD Pharmingen). (B) RT-PCR was used to assess the expression of FcγRIIb1/2 on U937 compared with B lymphocytes and dendritic cells. (C) FcγRI and FcγRIIa trigger differential cytosolic Ca2+ signals. Cytosolic calcium was measured in U937 by cuvette fluorimetry over 8 minutes after cross-linking of the individual FcRs using antibody clones 10.1 and 3D3 (BD Pharmingen), respectively (XL). (D) FcγRI triggers PLD activity, whereas FcγRIIa does not. PLD activity measured in resting U937 cells (Basal) or in cells after FcγR aggregation (XL FcγRI or XL FcγRIIa) for 30 minutes. (E) FcγRIIa triggers PLC activation in U937 cells. InsP3 generation was measured in resting cells (basal) or in cells after FcγR aggregation (XL FcγRI or XL FcγRIIa) for 15 minutes. (F) The PLC inhibitor U73122 blocks the FcγRIIa-mediated cytosolic calcium signal in U937 cells. Cells pretreated with the PLC inhibitor U73122 for 45 minutes were assayed for cytosolic calcium over 8 minutes after FcR cross-linking (XL FcγRI or XL FcγRIIa). (G) In IFN-γ–treated U937 (i-ii) and primary human monocytes (iii-iv), MEK1 and ERK1/2 are activated by FcγRI ligation by antibody clone 10.1 to a greater extent than by FcγRIIa ligation by antibody clone 3D3. Phosphoprotein array (Biorad) was used to measure ERK1/2 and MEK1 phosphorylation in cell lysates. MEK1, ERK, PLC, and PLD activity is expressed as a mean ± SD from 3 independent experiments. All intracellular calcium measurements were carried out in the presence of 1.5 M extracellular calcium and results shown are typical of 3 independent experiments.
Differential calcium signaling induced by FcγRI versus FcγRIIa
It has previously been shown that cross-linking FcγRI and FcγRII on U937 results in increases in intracellular calcium.15 We have previously extended these studies to show that in IFN-γ-treated U937, FcγRI induces coupling of phospholipase D with sphingosine kinase to trigger a single “spike” of calcium of relatively short duration, released from internal stores.16 Here, we examined whether the same mechanism is used by the low-affinity IgG receptor (FcγRIIa). In contrast to FcγRI, FcγRIIa triggers a calcium response for a longer duration (Figure 1C). Whereas FcγRI elicits a short-lived calcium response that lasts for 2 minutes, FcγRIIa triggers a response that lasts for more than 1 minute (Figure 1C). We next determined whether FcγRIIa is linked to the same lipid signaling pathways used by FcγRI. We investigated coupling of FcγRIIa to the PLD-SPHK pathway. As expected, FcγRI aggregation triggers PLD activity (Figure 1D). In contrast, FcγRIIa did not trigger a significant rise in PLD activation (Figure 1D). As no PLD activation was triggered by FcγRIIa aggregation, we determined whether the phospholipase C pathway triggers the observed calcium signals. Aggregation of FcγRIIa elicits a significant transient rise in InsP3 levels in U937 (Figure 1E). In contrast, no InsP3 could be detected after FcγRI aggregation (Figure 1E). Furthermore, an inhibitor of PLC, U73122, that blocks the calcium response triggered by FcγRIIa had no effect on FcγRI (Figure 1F). This confirms that the 2 receptors couple to different phospholipid signaling molecules to trigger intracellular calcium signals. Phosphoprotein array results also indicate that cross-linking FcγRI activates the MEK1/ERK pathway to a greater extent, compared with FcγRIIa in IFN-γ-treated U937. These observations can also be extended to primary human monocytes in which cross-linking FcγRI results in a significantly stronger induction of both phospho-MEK1 and phospho-ERK1/2 compared with FcγRIIa (Figure 1G).
FcγRIIa triggers the activation of PLCγ1
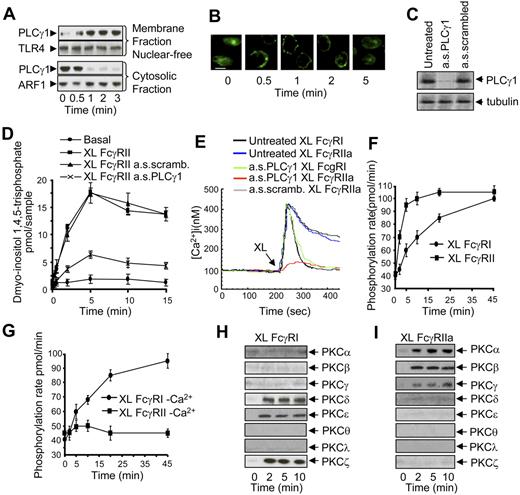
We investigated which isoform of PLC is involved in FcγRIIa signaling by immunoblot and fluorescence microscopy on cellular fractions. After FcγRIIa aggregation, PLCγ1 rapidly translocates to the nuclear-free membrane fraction, whereas no PLCγ1 could be detected in the membrane fraction prepared from resting cells (Figure 2A). Thus, 30 seconds after FcγRIIa aggregation, a band corresponding to the correct molecular weight for PLCγ1 was detected in the membrane fraction and remained associated with this fraction up to 5 minutes (30 seconds, 1 minute, 2 minutes, and 5 minutes; Figure 2A). These results were confirmed by fluorescence microscopy—in resting cells, PLCγ1 has a homogenous cytosolic distribution before FcγRIIa aggregation. After FcγRIIa aggregation, PLCγ1 redistributes to the cell periphery, exhibiting a plasma membrane localization (Figure 2B). Using antisense oligonucleotides specific for PLCγ1, we knocked down PLCγ1 expression levels (Figure 2C). When InsP3 and calcium responses were triggered by FcγRIIa, we observed that both signals were substantially reduced (Figure 2D and E, respectively), whereas FcγRI aggregation retained its ability to stimulate calcium responses (Figure 2E).
FcγRIIa triggers PLCγ1 activity, PLCγ1 dependent Ca2+ signals, and PKC activity in U937. (A) Immunoblot analysis was used to assay PLCγ1 translocation after FcγRIIa activation by antibody cross-linking in U937. A comparison is shown between a nuclear-free membrane-fraction (top panels) with the cytosolic fraction (bottom panels) probed with an anti-PLCγ1 antibody. TLR-4 and ARF were analyzed as loading controls for the membrane fraction and cytosolic fractions, respectively. (B) U937 stained for PLCγ1 after FcγRIIa cross-linking using antibody clone 3D3 over 5 minutes. Scale bar indicates 20 μm. (C) U937 pretreated with anti-PLCγ1 oligonucleotides down-regulate PLCγ1. Immunoblot analysis of untreated cells, treated cells (a.s.PLCγ1), and scrambled oligo (a.s.scrambled) as a control. α-Tubulin was used as a loading control. (D) FcγRIIa signals through PLCγ1. InsP3 generation was used as a readout for PLCγ1 activation. Basal controls compared with FcγRIIa activation (XL FcγRIIa) and with cells pretreated with antisense oligo (XL FcγRIIa a.s.PLCγ1) or scrambled oligo (XL FcγRIIa a.s.scramb.). (E) PLCγ1 activation by FcγRIIa is linked to cytosolic calcium signaling in U937. Cytosolic calcium signals in U937 were assayed by cuvette fluorimetry after FcR aggregation (untreated XL FcγRI or untreated XL FcγRIIa) compared with cells pretreated with antisense oligo against PLCγ1 (a.s.PLCγ1 XL FcγRI or a.s.PLCγ1 XL FcγRIIa) or a scrambled oligo control (a.s.scramb. XL FcγRIIa). (F) PKC activity in the presence of Ca2+ was measured as the phosphorylation rate (pmol/min) in samples from whole cell lysates after aggregation of FcγRI (labeled XL FcγRI) or FcγRIIa (XL FcγRIIa). (G) PKC activity in the absence of Ca2+ was similarly measured. (H) FcγRI-mediated PKC isoform translocation. Immunoblot analysis of different PKC isoenzymes translocated to the nuclear-free membrane fraction after antibody-mediated FcγRI aggregation over 10 minutes. (I) FcγRIIa-mediated PKC isoform translocation similarly measured in nuclear-free membrane fractions. Immunoblots and calcium traces shown are typical from at least 3 separate experiments. Graphic data represent mean ± SD from 3 experiments.
FcγRIIa triggers PLCγ1 activity, PLCγ1 dependent Ca2+ signals, and PKC activity in U937. (A) Immunoblot analysis was used to assay PLCγ1 translocation after FcγRIIa activation by antibody cross-linking in U937. A comparison is shown between a nuclear-free membrane-fraction (top panels) with the cytosolic fraction (bottom panels) probed with an anti-PLCγ1 antibody. TLR-4 and ARF were analyzed as loading controls for the membrane fraction and cytosolic fractions, respectively. (B) U937 stained for PLCγ1 after FcγRIIa cross-linking using antibody clone 3D3 over 5 minutes. Scale bar indicates 20 μm. (C) U937 pretreated with anti-PLCγ1 oligonucleotides down-regulate PLCγ1. Immunoblot analysis of untreated cells, treated cells (a.s.PLCγ1), and scrambled oligo (a.s.scrambled) as a control. α-Tubulin was used as a loading control. (D) FcγRIIa signals through PLCγ1. InsP3 generation was used as a readout for PLCγ1 activation. Basal controls compared with FcγRIIa activation (XL FcγRIIa) and with cells pretreated with antisense oligo (XL FcγRIIa a.s.PLCγ1) or scrambled oligo (XL FcγRIIa a.s.scramb.). (E) PLCγ1 activation by FcγRIIa is linked to cytosolic calcium signaling in U937. Cytosolic calcium signals in U937 were assayed by cuvette fluorimetry after FcR aggregation (untreated XL FcγRI or untreated XL FcγRIIa) compared with cells pretreated with antisense oligo against PLCγ1 (a.s.PLCγ1 XL FcγRI or a.s.PLCγ1 XL FcγRIIa) or a scrambled oligo control (a.s.scramb. XL FcγRIIa). (F) PKC activity in the presence of Ca2+ was measured as the phosphorylation rate (pmol/min) in samples from whole cell lysates after aggregation of FcγRI (labeled XL FcγRI) or FcγRIIa (XL FcγRIIa). (G) PKC activity in the absence of Ca2+ was similarly measured. (H) FcγRI-mediated PKC isoform translocation. Immunoblot analysis of different PKC isoenzymes translocated to the nuclear-free membrane fraction after antibody-mediated FcγRI aggregation over 10 minutes. (I) FcγRIIa-mediated PKC isoform translocation similarly measured in nuclear-free membrane fractions. Immunoblots and calcium traces shown are typical from at least 3 separate experiments. Graphic data represent mean ± SD from 3 experiments.
FcγRI and FcγRIIa activate different PKC isoforms
We have previously shown that FcγRI triggers a fast activation of PKC activity.18 When we compare the kinetics of PKC activation, we observed that FcγRI triggers an initial rise in PKC activity, followed by a sustained increase over a 45-minute period (Figure 2F). In contrast, FcγRIIa aggregation triggers a more rapid PKC response, reaching a plateau at 3 minutes, which is maintained over the 45-minute time course (Figure 2F).
Intracellular calcium has been proposed to be an important cofactor for PKC activity. However, we have previously shown that FcγRI triggers calcium-independent PKC activity.18 It has been shown that the amplitude and modulation of a calcium response can influence PKC isoform activation, so we investigated the calcium dependence of FcγRIIa-triggered PKC activation. As previously reported,18 the increase in PKC activity after FcγRI aggregation was unaffected by the withdrawal of calcium from the assay (Figure 2G). In contrast, withdrawal of calcium completely abolished any increase in PKC activity after FcγRIIa aggregation (Figure 2G). These results demonstrate that FcγRIIa triggers the activation of calcium-dependent PKC isoforms.
As PKC activation is associated with translocation to plasma membranes, we investigated whether FcγRI and FcγRIIa result in differential activation of particular PKC isoforms. Using subcellular fractionation, we show that FcγRI triggers the translocation of the calcium-independent PKCs δ, ϵ, and ζ isoforms from the cytosol to the plasma membrane (Figure 2H). In contrast, FcγRIIa aggregation induces the translocation of the calcium-dependent PKC α, β, and γ isoforms (Figure 2I). Taken together, these results show that there is a marked difference in the specific PKC isoforms activated by FcγRI versus FcγRIIa.
FcγRI-triggered PKC activity and isoform translocation are dependent on PLD1; FcγRIIa-triggered PKC activity and isoform translocation are dependent on PLCγ1
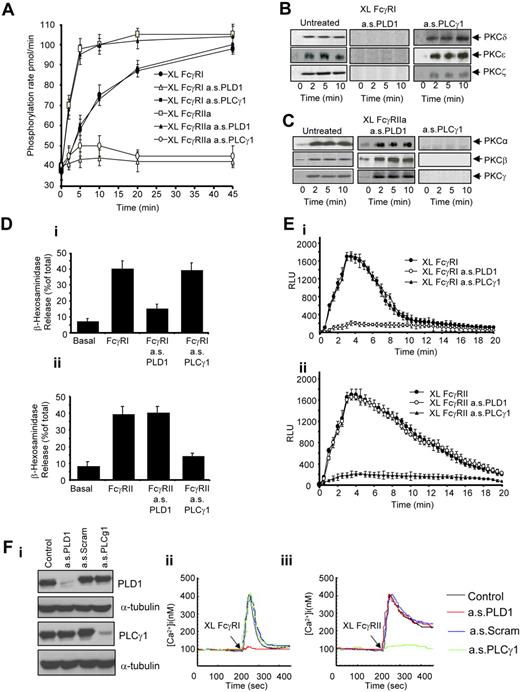
In cells pretreated with an antisense specific for PLD1, cross-linked FcγRI-triggered PKC activity and isoform translocation were substantially inhibited. No inhibition was observed in parallel U937 cells treated with antisense PLCγ1 (Figure 3A and B, respectively). When we cross-linked FcγRIIa in cells where PLCγ1 was knocked down, PKC enzyme activity and PKC isoform translocation were substantially reduced. No inhibition was observed in cells where PLD1 had been knocked down (Figure 3A and C, respectively).
Role of PLD1 and PLCγ1 in FcγRI- and FcγRIIa-mediated signaling. (A) FcγRI- and FcγRIIa-mediated PKC activity measured as the phosphorylation rate (pmol/min) in whole-cell lysates. Antibody-mediated FcγR aggregation in untreated cells (XL FcγRI (antibody 10.1) or XL FcγRIIa (antibody 3D3), cells pretreated with the antisense oligos against PLD1 (XL FcγRI a.s.PLD1 or XL FcγRIIa a.s.PLD1), or antisense oligos against PLCγ1 (XL FcγRI a.s.PLCγ1 or XL FcγRIIa a.s.PLCγ1).(B) FcγRI-mediated PKC isoform translocation requires PLD1. Immunoblot analysis of the PKC isoenzymes translocated to the nuclear-free membrane fraction after antibody-mediated FcγRI aggregation in control cells (untreated) and cells pretreated with the antisenseoligos against PLD1 or PLCγ1 (a.s.PLD1 or a.s.PLCγ1). (C) FcγRIIa-mediated PKC isoform translocation requires PLCγ1. The same analyses applied to cells after FcγRIIa activation. (D) FcγRI and FcγRIIa-mediated degranulation. (i) β-Hexosaminidase release in resting cells (basal) or after antibody-mediated FcγRI aggregation in control cells (XL FcγRI) compared with cells pretreated with antisense oligonucleotides to either PLD1 (XL FcγRI a.s.PLD1) or PLCγ1 (XL FcγRI a.s.PLCγ1). (ii) The same analyses applied to cells after FcγRIIa activation. (E) FcγRI and FcγRIIa-mediated activation of NADPH oxidative burst. (i) Superoxide production in response to antibody-mediated FcγRI activation in control cells (XL FcγRI) compared with cells pretreated with antisense oligos to either PLD1 (XL FcγRI a.s.PLD1) or PLCγ1 (XL FcγRI a.s.PLCγ1). (ii) The same analyses applied to cells after FcγRIIa activation. Results expressed are the mean ± SD of triplicate samples of 3 independent experiments. (Fi) Primary human monocytes pretreated with anti-PLD1 or anti-PLCγ1 oligonucleotides down-regulate PLD1 and PLCγ1, respectively, compared with α-tubulin controls. (Fii) Monocytes treated with anti-PLD1 oligonucleotides are impaired in their cytosolic calcium signaling after activation of FcγRI by receptor cross-linking using antibody clone 10.1. (Fiii) Monocytes treated with anti-PLCγ1 oligonucleotides are impaired in their cytosolic calcium signaling after activation of FcγRIIa by receptor cross-linking using antibody clone 3D3.
Role of PLD1 and PLCγ1 in FcγRI- and FcγRIIa-mediated signaling. (A) FcγRI- and FcγRIIa-mediated PKC activity measured as the phosphorylation rate (pmol/min) in whole-cell lysates. Antibody-mediated FcγR aggregation in untreated cells (XL FcγRI (antibody 10.1) or XL FcγRIIa (antibody 3D3), cells pretreated with the antisense oligos against PLD1 (XL FcγRI a.s.PLD1 or XL FcγRIIa a.s.PLD1), or antisense oligos against PLCγ1 (XL FcγRI a.s.PLCγ1 or XL FcγRIIa a.s.PLCγ1).(B) FcγRI-mediated PKC isoform translocation requires PLD1. Immunoblot analysis of the PKC isoenzymes translocated to the nuclear-free membrane fraction after antibody-mediated FcγRI aggregation in control cells (untreated) and cells pretreated with the antisenseoligos against PLD1 or PLCγ1 (a.s.PLD1 or a.s.PLCγ1). (C) FcγRIIa-mediated PKC isoform translocation requires PLCγ1. The same analyses applied to cells after FcγRIIa activation. (D) FcγRI and FcγRIIa-mediated degranulation. (i) β-Hexosaminidase release in resting cells (basal) or after antibody-mediated FcγRI aggregation in control cells (XL FcγRI) compared with cells pretreated with antisense oligonucleotides to either PLD1 (XL FcγRI a.s.PLD1) or PLCγ1 (XL FcγRI a.s.PLCγ1). (ii) The same analyses applied to cells after FcγRIIa activation. (E) FcγRI and FcγRIIa-mediated activation of NADPH oxidative burst. (i) Superoxide production in response to antibody-mediated FcγRI activation in control cells (XL FcγRI) compared with cells pretreated with antisense oligos to either PLD1 (XL FcγRI a.s.PLD1) or PLCγ1 (XL FcγRI a.s.PLCγ1). (ii) The same analyses applied to cells after FcγRIIa activation. Results expressed are the mean ± SD of triplicate samples of 3 independent experiments. (Fi) Primary human monocytes pretreated with anti-PLD1 or anti-PLCγ1 oligonucleotides down-regulate PLD1 and PLCγ1, respectively, compared with α-tubulin controls. (Fii) Monocytes treated with anti-PLD1 oligonucleotides are impaired in their cytosolic calcium signaling after activation of FcγRI by receptor cross-linking using antibody clone 10.1. (Fiii) Monocytes treated with anti-PLCγ1 oligonucleotides are impaired in their cytosolic calcium signaling after activation of FcγRIIa by receptor cross-linking using antibody clone 3D3.
Different signaling molecules are involved in FcγRI and FcγRIIa induced degranulation and oxidative burst
Intracellular calcium and PKC activity are linked to the release of granular contents (degranulation) and also to the NADPH-oxidative burst in U937.36,37 We investigated whether FcγRI and FcγRIIa cross-linking results in differences in these responses. In contrast to the differences observed in calcium signals and PKC activation, broadly similar levels of β-hexosaminidase release (degranulation) were triggered by FcγRI and FcγRIIa (Figure 3Di and ii). We also observed that FcγRI-triggered β-hexosaminidase release is dependent on PLD1 and not PLCγ1 (Figure 3Di). In contrast, FcγRIIa-triggered β-hexosaminidase release was dependent on PLCγ1 and not PLD1 (Figure 3Dii). Moreover, the activation of the NADPH-oxidative burst exhibited different kinetics between the 2 receptors (Figure 3Ei and ii). FcγRI-triggered NADPH-activity depends on PLD1 (Figure 3Ei), whereas the FcγRIIa-triggered oxidative burst depends on PLCγ1 (Figure 3Eii). These results suggest that although the cellular functions of degranulation and oxidative burst are triggered by either receptor, the nature and kinetic of the signals that induce these functional responses are different.
We tested whether the link between FcγRI and PLD1 and PLCγ1 and FcγRIIa signaling in U937 could also be extended to primary human monocytes. In primary monocytes treated with antisense specific for PLD1 or antisense PLCγ1, a significant reduction in PLD1/PLCγ1 expression levels was observed (Figure 3Fi). In monocytes treated with antisense PLD1, a significant reduction in induced cytosolic calcium was observed after antibody-mediated aggregation of FcγRI. In contrast, when FcγRIIa was activated, the cytosolic calcium response was inhibited in cells treated with antisense PLCγ1. As with earlier observations on MEK1 and ERK1/2, these data suggest that the signaling activity associated with FcγRI and FcγRIIa in IFN-γ-treated U937 can be extended to primary human monocytes (Figure 3Fii and iii).
FcγRI and FcγRIIA triggered secretion of cytokines and chemokines
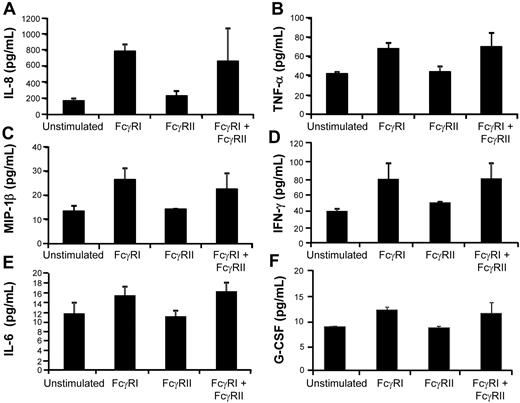
U937 cells were stimulated by cross-linking either FcγRI and/or FcγRIIa for 10 minutes and culture supernatants harvested 24 hours later. Supernatants were assayed for cytokine secretion with a Bio-Rad 17-plex cytokine array, and we observed that the proinflammatory cytokines and chemokines IL-8, TNF-α, MIP-1β, IL-6, and G-CSF were differentially expressed/secreted between the 2 cross-linked Fc receptors in 4 independent experiments, with triggering of FcγRI inducing higher levels of secretion. (Figure 4A-F).
Differential cytokine/chemokine release triggered by FcγRI versus FcγRIIa activation. U937 cells were cross-linked with anti-CD32 (antibody 3D3) and/or anti-CD64 (antibody 10.1) plus goat anti–mouse IgG F(ab)′2 for 10 minutes and cells were placed in culture for 24 hours. Culture supernatants were harvested 24 hours later and assayed for 17 different cytokine targets by Bioplex cytokine array analysis. Higher levels of (A) IL-8, (B) TNF-α, (C) MIP-1β, (D) IFN-γ, (E) IL-6, and (F) G-CSF were secreted after cross-linking of FcγRI versus FcγRIIa. Results are expressed as the mean ± SD of triplicate samples from 4 independent experiments.
Differential cytokine/chemokine release triggered by FcγRI versus FcγRIIa activation. U937 cells were cross-linked with anti-CD32 (antibody 3D3) and/or anti-CD64 (antibody 10.1) plus goat anti–mouse IgG F(ab)′2 for 10 minutes and cells were placed in culture for 24 hours. Culture supernatants were harvested 24 hours later and assayed for 17 different cytokine targets by Bioplex cytokine array analysis. Higher levels of (A) IL-8, (B) TNF-α, (C) MIP-1β, (D) IFN-γ, (E) IL-6, and (F) G-CSF were secreted after cross-linking of FcγRI versus FcγRIIa. Results are expressed as the mean ± SD of triplicate samples from 4 independent experiments.
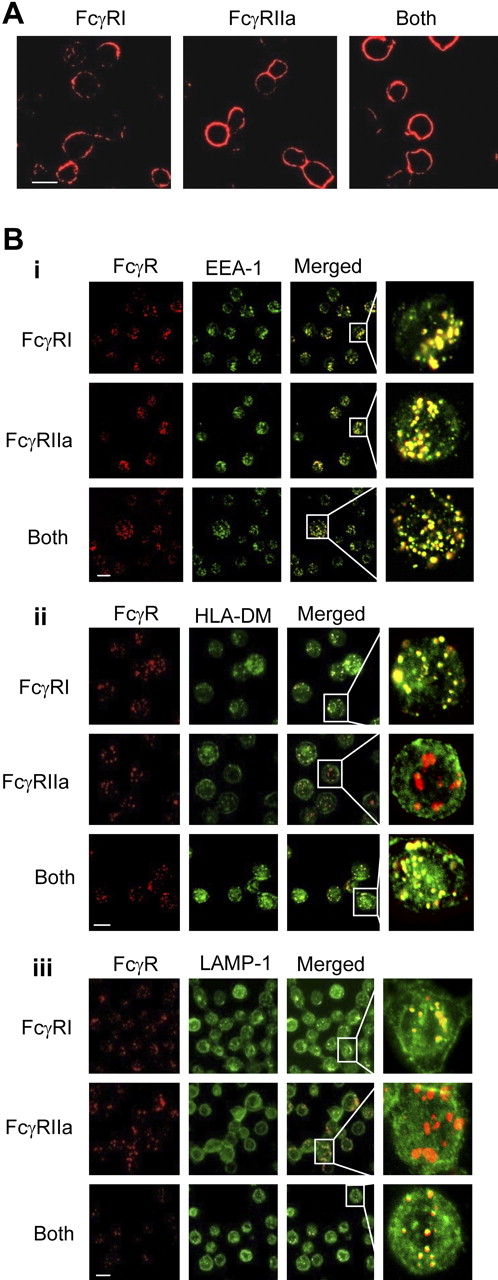
Differential membrane trafficking of FcγRI and FcγRIIa receptors
In the absence of multivalent ligand, FcγRI, and FcγRIIa are found on the cell surface of U937 and primary monocytes (Figure 5A). Antibody-mediated aggregation induces FcγRI and FcγRIIa molecules to enter intracellular compartments expressing the early endosomal marker EEA-1 (Figure 5Bi) and the SNARE proteins Syntaxin 4 and TI-VAMP (see supplemental Figure 2Ai-ii). The FcγRI-containing endosome exhibits positive staining for human leukocyte antigen (HLA)–DM. HLA-DM is a putative marker for MIIC antigen-processing compartments, in which major histocompatibility complex (MHC) class II molecules are loaded with peptides derived from endocytosed antigens.38,39 In contrast, cross-linked FcγRIIa internalizes into compartments that show little or no costaining for HLA-DM over an identical period (Figure 5Bii). The endosomal compartment containing the FcγRI receptor also exhibits a significant degree of staining for the late endosomal/lyzosomal marker LAMP-1. FcγRIIa positive compartments exhibited little or no colocalization with this marker over an identical period (Figure 5Biii).
Differential membrane trafficking of FcγRI and FcγRIIa receptors. FcγRI or FcγRII were cross-linked with mouse anti–human CD64 (antibody 10.1) and/or mouse anti–human CD32 (antibody 3D3), respectively. The secondary monoclonal used for cross-linking the specific mAbs in these experiments was an Alexa Fluor 647–conjugated goat anti–mouse IgG F(ab)′2 (Invitrogen). Receptors were cross-linked for 10 minutes at 37°C to allow internalization. At this time point, cells were fixed and frozen in methanol/acetone (1:1) at −20°C for at least 24 hours before further staining. (A) In the absence of FcR cross-linking, FcγRI and FcγRIIa remain localized at the cell surface. (B) After FcR cross-linking for 10 minutes, (i) both receptors enter a compartment that stains positive for the early endosome marker EEA-1. However, whereas FcγRI enters a compartment that also stains positive for (ii) HLA-DM and (iii) LAMP-1, FcγRIIa enters a compartment that is negative for both these markers of MIIC compartments. Scale bar indicates 20 μm. Results shown are typical of at least 3 separate experiments. Anti–EEA-1 and anti-LAMP1 polyclonal antibodies were purchased from Santa Cruz. Rabbit anti–HLA-DM was provided by Dr Adrian Kelly (Immunology Unit, Department of Pathology, Cambridge University, Cambridge, United Kingdom).
Differential membrane trafficking of FcγRI and FcγRIIa receptors. FcγRI or FcγRII were cross-linked with mouse anti–human CD64 (antibody 10.1) and/or mouse anti–human CD32 (antibody 3D3), respectively. The secondary monoclonal used for cross-linking the specific mAbs in these experiments was an Alexa Fluor 647–conjugated goat anti–mouse IgG F(ab)′2 (Invitrogen). Receptors were cross-linked for 10 minutes at 37°C to allow internalization. At this time point, cells were fixed and frozen in methanol/acetone (1:1) at −20°C for at least 24 hours before further staining. (A) In the absence of FcR cross-linking, FcγRI and FcγRIIa remain localized at the cell surface. (B) After FcR cross-linking for 10 minutes, (i) both receptors enter a compartment that stains positive for the early endosome marker EEA-1. However, whereas FcγRI enters a compartment that also stains positive for (ii) HLA-DM and (iii) LAMP-1, FcγRIIa enters a compartment that is negative for both these markers of MIIC compartments. Scale bar indicates 20 μm. Results shown are typical of at least 3 separate experiments. Anti–EEA-1 and anti-LAMP1 polyclonal antibodies were purchased from Santa Cruz. Rabbit anti–HLA-DM was provided by Dr Adrian Kelly (Immunology Unit, Department of Pathology, Cambridge University, Cambridge, United Kingdom).
We determined whether the membrane trafficking events observed in U937 are also found in primary FcR-expressing dendritic cells and monocytes. As in U937, when FcγRI is cross-linked, it enters a compartment that stains for both the early endosomal marker EEA-1 (see supplemental Figure 2Bi and ii, respectively) and late endosome/lysosome marker LAMP-1 (see supplemental Figure 2Biii and iv, respectively). FcγRIIa enters a compartment that costains for the early endosome marker EEA-1 (see supplemental Figure 2Bi and ii, respectively) but not LAMP-1 (see supplemental Figure 2Biii and iv, respectively).
Antigen presentation of FcγRI and FcγRIIa internalized immune complexes
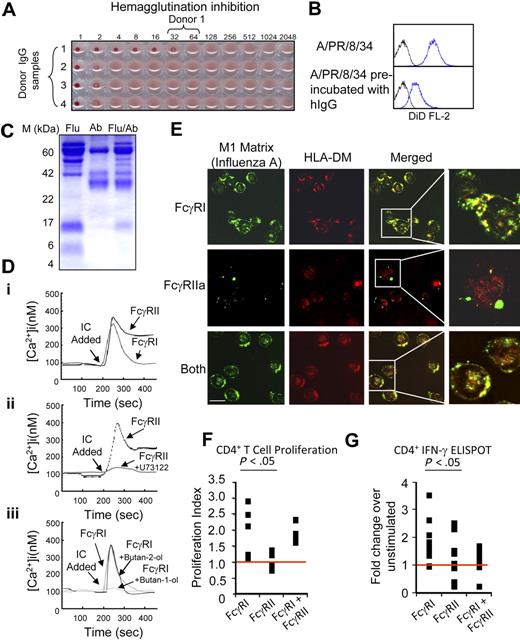
We sought to determine whether differences in signaling and intracellular trafficking mediated by FcγRI and FcγRIIa impacts upon antigen presentation. Influenza A virus was used as a source of antigens for these experiments. This is an orthomyxovirus that encodes several well-characterized antigenic determinants.40,41 To generate human IgG against the influenza A virus, healthy human volunteers were vaccinated with the current seasonal influenza vaccine and then bled 21 to 60 days postinoculation. IgG was purified from serum samples by fast performance liquid chromatography. Hemagglutination inhibition was used to screen the purified human IgGs for binding activity to influenza A/PR/8/34 (Figure 6A). In addition, we screened the neutralizing activities of purified hIgG to influenza A/PR/8/34, by incubating live influenza A virus with the lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine (DiD),42 then demonstrating a reduction in labeled virus binding to U937 mediated by hIgG (Figure 6B). Human IgG fractions with clear binding activity were used in conjunction with influenza A/PR/8/34 to construct in vitro immune complexes, enriched and purified using a Vivaspin micro-concentrator system. Purified IC containing protein bands derived from hIgGs and virus were visualized on Coomassie-stained SDS-PAGE gels (Figure 6C).
Antigen/immune complexes internalized through FcγRI are presented more efficiently to CD4+ T cells. (A) A hemagglutination inhibition assay was used on serum samples from a panel of influenza A–vaccinated human donors to identify samples contained high levels of anti–influenza A (strain A/PR/8/34) hIgG. (B) Flow cytometric analysis of live influenza A virus labeled with the lipophilic fluorescent dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine binding to U937 cells (top histogram); 1 μM of hIgG isolated from donor 1 inhibits the binding of the live virus (bottom histogram). (C) Purified immune complexes (IC) generated by incubating live influenza A virus with hIgG from donor 1 (Flu/Ab) exhibit protein bands from both the virus (Flu) and hIgG (Ab). (Di) Cytosolic calcium signaling in primary human monocytes stimulated with IC. IC-mediated FcγRI Ca2+ signaling was analyzed in the presence of a blocking antibody to FcγRII, IV.3. FcγRIIa signaling was analyzed in presence of excess hIgG to ensure receptor occupancy and blockade of FcγRI. (Dii) IC-mediated FcγRIIa induced cytosolic Ca2+ in primary monocytes treated with the PLC-γ inhibitor U73122 versus untreated controls. (Diii) IC-mediated FcγRI induced cytosolic Ca2+ in primary monocytes treated with the PLD inhibitor butan-1-ol (0.3%) versus butan-2-ol (0.3%) and untreated controls. (E) Top panel: FcγRIIa is blocked with antibody IV.3 to allow for FcγRI-mediated internalization of IC into primary human monocytes into enodocytic compartments that stain positive for HLA-DM, the putative MIIC marker, and the influenza A matrix protein (M1 matrix). Middle panel: FcγRI is blocked with excess hIgG to allow for IC internalization via FcγRIIa. Bottom panel: IC uptake via both FcγRI and FcγRIIa in the absence of receptor blockade. (F) Human monocytes that internalize influenza A/hIgG IC via FcγRI versus FcγRIIa stimulate greater proliferation in influenza-A-specific CD4+ human T lymphocyte cell lines; 5 cell lines derived from 3 unrelated human donors were tested with syngeneic monocytes pulsed with IC for 10 minutes in the presence or absence of FcγRI or FcγRIIa-blocking antibodies. 3H-Thymidine incorporation was used to measure cell proliferation and the resulting data expressed as a proliferation index. (G) ELISPOT analyses of influenza-A-specific CD4 T-cell responses in primary human cells. Monocytes and CD4+ T cells were enriched by CD8 depletion before internalization of IC via FcγRI and/or FcγRIIa. More than 9 independent experiments were carried out on unrelated human donors, the numbers of resulting ELISPOTs were assayed from 14 to 18 hours poststimulation using a CTL ImmunoSpot S4 analyzer. The fold difference between the number of spots per treatment condition over controls was calculated. An ANOVA analysis, followed by a protected t test, was conducted between the different conditions used for the proliferation assay and the IFN-γ ELISPOT, and the significance levels are as reported. All intracellular calcium measurements were carried out in the presence of 1.5 M extracellular Calcium and results shown are typical of 3 independent experiments.
Antigen/immune complexes internalized through FcγRI are presented more efficiently to CD4+ T cells. (A) A hemagglutination inhibition assay was used on serum samples from a panel of influenza A–vaccinated human donors to identify samples contained high levels of anti–influenza A (strain A/PR/8/34) hIgG. (B) Flow cytometric analysis of live influenza A virus labeled with the lipophilic fluorescent dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine binding to U937 cells (top histogram); 1 μM of hIgG isolated from donor 1 inhibits the binding of the live virus (bottom histogram). (C) Purified immune complexes (IC) generated by incubating live influenza A virus with hIgG from donor 1 (Flu/Ab) exhibit protein bands from both the virus (Flu) and hIgG (Ab). (Di) Cytosolic calcium signaling in primary human monocytes stimulated with IC. IC-mediated FcγRI Ca2+ signaling was analyzed in the presence of a blocking antibody to FcγRII, IV.3. FcγRIIa signaling was analyzed in presence of excess hIgG to ensure receptor occupancy and blockade of FcγRI. (Dii) IC-mediated FcγRIIa induced cytosolic Ca2+ in primary monocytes treated with the PLC-γ inhibitor U73122 versus untreated controls. (Diii) IC-mediated FcγRI induced cytosolic Ca2+ in primary monocytes treated with the PLD inhibitor butan-1-ol (0.3%) versus butan-2-ol (0.3%) and untreated controls. (E) Top panel: FcγRIIa is blocked with antibody IV.3 to allow for FcγRI-mediated internalization of IC into primary human monocytes into enodocytic compartments that stain positive for HLA-DM, the putative MIIC marker, and the influenza A matrix protein (M1 matrix). Middle panel: FcγRI is blocked with excess hIgG to allow for IC internalization via FcγRIIa. Bottom panel: IC uptake via both FcγRI and FcγRIIa in the absence of receptor blockade. (F) Human monocytes that internalize influenza A/hIgG IC via FcγRI versus FcγRIIa stimulate greater proliferation in influenza-A-specific CD4+ human T lymphocyte cell lines; 5 cell lines derived from 3 unrelated human donors were tested with syngeneic monocytes pulsed with IC for 10 minutes in the presence or absence of FcγRI or FcγRIIa-blocking antibodies. 3H-Thymidine incorporation was used to measure cell proliferation and the resulting data expressed as a proliferation index. (G) ELISPOT analyses of influenza-A-specific CD4 T-cell responses in primary human cells. Monocytes and CD4+ T cells were enriched by CD8 depletion before internalization of IC via FcγRI and/or FcγRIIa. More than 9 independent experiments were carried out on unrelated human donors, the numbers of resulting ELISPOTs were assayed from 14 to 18 hours poststimulation using a CTL ImmunoSpot S4 analyzer. The fold difference between the number of spots per treatment condition over controls was calculated. An ANOVA analysis, followed by a protected t test, was conducted between the different conditions used for the proliferation assay and the IFN-γ ELISPOT, and the significance levels are as reported. All intracellular calcium measurements were carried out in the presence of 1.5 M extracellular Calcium and results shown are typical of 3 independent experiments.
Purified IC were used to stimulate cytosolic calcium responses via FcγRI in monocytes treated with the FcγRIIa-blocking antibody, IV.3, or via FcγRIIa in monocytes treated with monomeric hIgGs (to block FcγRI). The form and duration of the calcium responses engendered were similar to those observed using antibody cross-linking in U937 and primary monocytes (Figure 6Di). Pretreatment of the primary monocytes with the PLC inhibitor U73122 blocked IC-induced responses via FcγRIIa, and the PLD inhibitor butan-1-ol blocked IC induced responses via FcγRI. These data suggest that the signaling pathways identified by antibody-cross-linking specific FcRs apply when physiologic stimuli such as human IC are used (Figure 6Dii-iii). Primary human monocytes that do not express FcγRIII were selected for these studies (see supplemental Figure 3C).
We addressed whether immune complexes delivered FcγRI versus FcγRIIa traffic to the same intracellular compartments as those observed with antibody-mediated receptor aggregation. Stained influenza antigens can be detected in HLA-DM+ MIIC compartments when the complexes are internalized via FcγRI in monocytes (IV.3 treated), whereas little or no colocalization is detected when the complexes are internalized via FcγRIIa (monomeric IgG treated; Figure 6E).
Five influenza-specific CD4+ T-lymphocyte cell lines were used to address the natural presentation of influenza antigens by proliferation assay. Influenza-specific CD4+ T cells were stimulated by syngeneic monocytes pulsed with immune complexes through FcγRI (IV.3 treated) or through FcγRIIa (monomeric IgG treated). With 4 of 5 of these influenza-specific cell lines, immune complexes internalized via FcγRI stimulated significantly higher levels of proliferation than those internalized through FcγRIIa (see supplemental Figure 3A). When these data are combined, we see a small but significant increase in the influenza-specific T-cell proliferation index as determined by ANOVA analysis combined with a Tukey protected t test (P < .05; Figure 6E).
ELISPOT analysis of CD4+ T-cell responses in primary human blood samples further confirm the dominance of FcγRI-mediated antigen presentation versus FcγRIIa, with 7 of 9 donors showing a significant enhancement via FcγRI and 2 of 9 showing either no preference or a small enhancement via FcγRIIa (see supplemental Figure 3B). When these data are combined and fold-differences in ELISPOT numbers are compared, a significant increase is observed when IC are internalized via FcγRI (P < .05; Figure 6F). These data suggest that MHC class II presentation of influenza epitopes is more effective when immune complexes enter antigen-presenting cells through FcγRI versus FcγRIIa.
Taken together, our results suggest fundamental differences in the molecular mechanisms used by FcγRI and FcγRIIa in monocytic cells that couple distinct signaling pathways to functional differences in their cell biology and immunology.
Discussion
The data presented in this paper demonstrate fundamental differences in intracellular signaling pathways, receptor trafficking, and antigen processing/presentation activities stimulated by the high-affinity activatory receptor for IgG (FcγRI) versus the low-affinity activatory receptor for IgG (FcγRIIa). Thus, in the IFN-γ-treated monocytic cell line U937, FcγRI couples to phospholipase D1 and sphingosine kinase 1 to trigger calcium release from internal stores. In contrast, aggregation of FcγRIIa leads to the activation of phospholipase Cγ1 to elicit calcium release. Moreover, we show that the PKC enzyme activity and isoform translocation induced by FcγRI is dependent on phospholipase D1, whereas responses triggered by FcγRIIa depend on the presence of phospholipase Cγ1. Similarly, degranulation mediated by FcγRI was dependent on PLD1, whereas the degranulation elicited through FcγRIIa was dependent on PLCγ1. There was no observed overlap in the utilization of the different phospholipases by either receptor.
The finding that FcγRI and FcγRIIa activate different intracellular signaling molecules to trigger calcium signals and other responses may have profound implications for their functional activities. It is likely that FcγRIIa produces a prolonged response to immune complex activation by using InsP3 and inducing calcium entry to allow store refilling, thereby maintaining the calcium signal. This switch in phospholipase activation and calcium signaling results in the differential activation of protein kinase C isoforms, which are involved in several myeloid functions, including phagocytosis.43-46 Differential protein kinase C signaling may explain the observation that FcγRI-triggered phagocytosis is calcium independent, whereas FcγRII-mediated phagocytosis requires a sustained rise in intracellular calcium. FcγRI and FcγRIIa aggregation stimulate the MEK1/ERK pathway, but FcγRI activates this pathway to a greater extent.
When we compared secreted cytokines and chemokines induced by FcR cross-linking, FcγRI activation elicits the production of higher levels of the proinflammatory mediators TNF-α, IL-8, IL-6, IFN-γ, G-CSF, and MIP-1β. This suggests that FcγRI may play a dominant role over FcγRIIA in the augmentation of inflammation and immunity. This conclusion is supported by data on membrane trafficking and antigen presentation. Immune complexes internalized via FcγRI traffic to membrane-processing compartments termed MIICs, in which antigens are broken down into short peptides for loading onto MHC class II molecules.47 When we induce the internalization of FcγRI or FcγRIIa in U937, we observe some overlap in staining between FcγRI and FcγRIIa, particularly in the early endocytic markers EEA-1, TI-VAMP, and syntaxin 4. However, over an identical period, FcγRIIa traffics to compartments that are negative for HLA-DM or LAMP-1, suggesting that they do not traffic to MIICs.
The extension of these observations to primary monocytes and dendritic cells is complicated by the presence of an additional FcγR. FcγRIIb is an immune-tyrosine inhibitory motif-containing inhibitory FcγR that regulates the signaling activity of other activatory FcRs. It has been shown that FcγRIIb expression in primary monocytes taken from healthy individuals is restricted to a small subset of cells.48 Our studies have shown that the signaling and membrane-trafficking events observed in U937 also apply to primary human monocytes, suggesting that the signaling pathways described here remain applicable to primary cells despite the possible coexpression of FcγRIIb on a small subset. In primary monocytes, complexes internalized via FcγRI localize to MIICs, whereas those internalized via FcγRIIa do not. This results in a marked difference in the ability of the monocytes to present antigens, derived from the immune complexes to influenza-A–specific CD4+ T lymphocytes. FcγRI exhibits significant dominance over FcγRIIa in the presentation of internalized antigens.
An additional complicating factor in our interpretation of the signaling activity of FcγRI is the possibility that mouse antibodies used to cross-link FcγRI coengage FcγRIIa on the surface of the cells. Based on our current antibody cross-linking methodologies, it is impossible to rule this out. However, it is also clear from our studies on immune-complex-mediated stimulation of primary human monocytes in which FcγRIIa is blocked with IV.3 that FcγRI signaling overlaps with our observations on antibody cross-linking, suggesting that coengagement of FcγRIIa is not a significant factor.
The physiologic significance of the distinct signaling pathways and antigenic fates subserved by FcγRI and FcγRIIa remains to be determined. Although the 2 receptors are often expressed on the same cell type, their ratios differ on different cells and may be further altered by differential regulation by cytokines, etc, in diverse immunologic contexts. Differential signaling between the two could also be influenced by the affinities of each receptor for distinct IgG isotypes, which are known to vary widely between immune responses (eg, T-dependent versus T-independent, or TH1 vs TH2). For example, only FcγRIIa binds IgG2, and autoimmunity-associated polymorphisms in FcγRIIa alter isotype affinity and have significant functional implications.49
Finally, the effect of the inhibitory FcγRIIb, itself tightly regulated, might favor signaling through FcγRI or FcγRIIa in different circumstances. Thus, summation of effects of the different IgG isotypes produced during immune responses, along with the regulation of FcRs on the different cell types, could harness the distinct signaling pathways we have described for FcγRI and FcγRIIa to control antigenic fate and immune outcome in health and disease.
Taken together, these data suggest fundamental differences in the activities of the 2 FcRs, with important implications for inflammation and immune homeostasis. FcγRI uptake and signaling primes human immune cells to enhance inflammation and antigen presentation, whereas FcγRIIa does not. New therapeutic strategies aimed at inducing an absolute switch in the activation of signaling pathways triggered by FcγRI or FcγRIIa may also provide the means to control the nature of potentially harmful or protective immune responses in infection and autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr. Adrian Kelly (Cambridge University, United Kingdom) for providing the rabbit anti-HLA DM used in this study. We also thank A.-K. Fraser-Andrews for proofreading the manuscript.
This work was supported by a Singapore-MIT Alliance for Research and Technology (SMART-MIT) Pilot project grant and Biomedical Research Council (BMRC) and Office of Life Sciences (OLS)–Young Investigator awards.
Authorship
Contribution: X.D., M.J., H.K.T., R.R., G.L., C.T.T., and Y.T.L. performed experiments; X.D., M.J., and H.K.T. analyzed results; X.D., M.J., and H.K.T. made the figures; R.A.F., K.G.C.S., S.H.C., and D.M.K. provided reagents and support for the research; and X.D., A.J.M., and P.A.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul A. MacAry, Immunology Program, Department of Microbiology, Center for Life Sciences, Singapore 129793; e-mail: micpam@nus.edu.sg; or Alirio J. Melendez, Division of Immunology, Infection and Inflammation, Glasgow Biomedical Research Center, University of Glasgow, Glasgow G12 8TA, United Kingdom; email: a.melendez-romero@clinmed.gla.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal