Abstract
Lymphoma idiotype protein vaccines have shown therapeutic potential in previous clinical studies, and results from a completed pivotal, phase 3 controlled trial are promising. However, streamlined production of these patient-specific vaccines is required for eventual clinical application. Here, we show that second-generation, chemokine-fused idiotype DNA vaccines, when combined with myotoxins that induced sterile inflammation with recruitment of antigen-presenting cells at vaccination sites, were exceptional in their ability to provoke memory antitumor immunity in mice, compared with several TLR agonists. The combined vaccination strategy elicited both antigen-specific T-cell responses and humoral immunity. Unexpectedly, vaccine-induced tumor protection was intact in B cell–deficient mice but was abrogated completely by T-cell depletion in vivo, suggesting T-cell dependence. Furthermore, the optimal effect of myotoxins was observed with fusion vaccines that specifically targeted antigen delivery to antigen-presenting cells and not with vaccines lacking a targeting moiety, suggesting that the rational vaccine design will require combination strategies with novel, proinflammatory agents and highly optimized molecular vaccine constructs. These studies also challenge the paradigm that antibody responses are the primary of idiotype-specific antitumor effects and support the optimization of idiotype vaccines designed to induce primarily T-cell immunity.
Introduction
Vaccine therapy, with the potential for eradicating residual disease and establishing long-term memory immunity to prevent relapses, may be a promising treatment for hematologic malignancies. A successful vaccine strategy relies largely on the selection of a tumor-specific antigen, against which immune responses specifically target tumor cells while sparing normal tissues. Idiotype has been identified as the unique sequences embedded in the variable regions of the heavy and light chains of the immunoglobulin molecule expressed on the surface of B cells. Given that malignancies of mature and resting B cells arise from clonal proliferation of cells that express immunoglobulins with a unique variable region sequence, the idiotype of a given B-cell malignancy can serve as a tumor-specific antigen and has been exploited as a target for vaccine therapy.1-3 Kwak et al4 initially evaluated individualized idiotype protein vaccines in human patients with lymphoma, and several other clinical trials of patients with lymphoma or myeloma have confirmed immunogenicity.5-7 Among the most important findings of these studies was the demonstration that in patients with follicular lymphoma in minimal residual disease, as defined by complete remission, autologous idiotype protein can be formulated into an immunogenic antigen when it is conjugated with a carrier protein, keyhole-limpet hemocyanin, and administrated with granulocyte-macrophage colony-stimulating factor as an adjuvant.5 In particular, the National Cancer Institute phase 2 clinical trial that used this vaccination strategy resulted in molecular remissions in most patients whose lymphoma could be detected by a characteristic Bcl-2 translocation and long-term disease-free survival in first complete remission.5 The encouraging results from this phase 2 study led to pivotal, phase 3 controlled randomized clinical trials, one of which recently reported promising results.8
The current technique of producing patient-specific, hybridoma-generated idiotype protein is labor intensive. Accordingly, the development of second-generation idiotype vaccines, which simplifies the manufacturing process of idiotype proteins, is needed. To achieve these goals, efforts have been made to develop DNA vaccines that encode a short idiotype single-chain (sFv) polypeptide containing only the antigenic variable regions of light and heavy chains. Further modification of idiotype sFv for targeted antigen delivery strategies to specialized antigen-presenting cells (APCs) was observed to induce protective immunity in mouse models against lymphoma and myeloma,9,10 which highlighted the potential of plasmid DNA encoding genetically modified idiotype sFv as next-generation idiotype vaccines.
However, given that plasmid DNA is an inherently weak immunogenic, rational design of idiotype DNA vaccines should include combination strategies with other agents that can potentiate immunogenicity. The few adjuvants available for use in humans often induce suboptimal protective immunity.11,12 Recent reports suggest that various cellular elements of the innate immune system such as endogenous ligands of toll-like receptors are released after tissue necrosis.13 Given that the induction of robust adaptive immune responses requires intact innate immunity, components of the innate immune system are important for shaping the quality of adaptive immune responses. For example, TLR agonists, functioning through pattern recognition receptors on APCs, improve the potency of antigen-specific T-cell responses elicited by vaccines.14-16 Thus, we reason that the combination of vaccine therapy with such molecular adjuvants is critical for optimizing adaptive immune responses resulting from idiotype DNA vaccines.
Severe tissue necrosis and inflammation have long been noted in persons surviving from a viper bite. Cardiotoxin, a small myotoxic polypeptide present in viper venom, has been recognized as the principal component responsible for tissue necrosis and inflammation. Although its name implies that it is toxic to the heart, intramuscular injection of mice with cardiotoxin was shown to induce a local tissue necrosis–muscle regeneration cycle without cardiotoxic effects.17 In this study, we demonstrated that idiotype DNA vaccine-triggered therapeutic effects were significantly enhanced by pretreating the vaccination sites with a low dose of cardiotoxin. Cardiotoxin causes inflammatory infiltration, triggering the recruitment of APCs, which in turn facilitates the activation of idiotype-specific adaptive immunity, including memory. Using B cell–deficient mice, we demonstrated for the first time that anti-idiotype antibody response was not essential for the eradication of lymphoma cells; instead, T-cell immunity played the critical role in vaccine-induced tumor protection.
Methods
Cell line and animals
A20 murine lymphoma cells were expanded in large scale, and aliquots were frozen down from the common passage used for the animal experiments described thereafter. A reproducible tumor titration on this passage of A20 cells was performed with groups of 10 Balb/c mice to identify the minimal lethal dose that was found to be 2 × 105 cells/mice. Balb/c mice were obtained from the National Cancer Institute. Jh (Balb/c background) mice were purchased from Taconic Farms. All mice were maintained in a pathogen-free mouse facility according to institutional guidelines. All animal studies were approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center.
Adjuvants and immunostimulants
Cardiotoxin and crotoxin were kindly provided by Dr Paul Reid (Recptopharm Inc). For treatment, 6.8 μg cardiotoxin17,18 or 0.1 μg crotoxin (dose recommended by the manufacturer) was injected into quadriceps 5 days before vaccination. At those doses, the myotoxins induced muscle degeneration–regeneration without causing significant systemic toxicity in mice. The Toll-like receptor agonists Poly I:C (TLR3 agonist), MPL (TLR4 agonist; InvivoGen), M001 (TLR7 agonist), and M003 (TLR7/8 agonist; 3M Company) were given, respectively, on the day after vaccination at doses of 50 μg.19,20
Constructs and vaccination
Plasmid DNA constructs encoding MCP3-sFv, MIP3α-sFv, and Defensin2β-sFv, respectively, were generated in our early studies.10,21,22 Ova full-length cDNA was cloned from ova-expressing B16 melanoma cells by reverse transcription–polymerase chain reaction and genetically fused with MCP3. In prophylactic vaccination studies, groups of 10 mice were anesthetized and intramuscularly injected with 50 μg plasmid DNA. A total of 3 vaccinations were given on days 0, 14, and 28. Cardiotoxin or crotoxin was given during the first 2 rounds of vaccination. Two weeks after final vaccination, a lethal dose of 2 × 105 A20 lymphoma cells was given by intraperitoneal injection. Tumor-free mice surviving the prophylactic studies were rechallenged with 2 × 105 A20 cells and followed for survival for 80 days. In therapeutic studies, mice were challenged with 2 × 105 A20 tumor cells on day 0 followed by vaccinations on days 1, 4, 8, and 18.21 Cardiotoxin was given 5 days before the first and final vaccinations. In all animal studies, data were statistically analyzed using the Kaplan-Meier method with a log-rank P value.
In vivo T-cell depletion
T-cell depletion was performed by intraperitoneal injection of 200 μg monoclonal antibody against CD8 (clone 2.43) and/or CD4 (clone GK1.5) as the schedule shows in Figure 4. The efficiency of T-cell depletion was assessed by staining peripheral blood mononuclear cells with CD4-PE, CD8-FITC, and CD3-APC (BD Biosciences).
Idiotype-specific immune responses
T cell–mediated immunity was assessed by examining the activation of idiotype-specific and tumor-reactive T cells. The immunized splenocytes were isolated and were in vitro activated for 5 days with bone marrow–derived dendritic cells (DCs) pulsed with A20 sFv H-2Kd epitope peptide (A20106-114) at a concentration of 5 μg/mL.23 The stimulated splenocytes were then seeded in a 96-well enzyme-linked immunospot (ELISPOT) plate at 2.5 × 105 cells/well either with the peptides or with irradiated A20 tumor cells at a 5:1 T cell/stimulator ratio for 48 hours.24 IFN-γ–producing T cells were detected using an IFN-γ ELISPOT kit (BD Biosciences) and were analyzed on a CTL ImmunoSpot Analyzer (Cellular Technology Ltd). The Student t test was used for statistical analysis. The antibody response was determined by measuring serum levels of anti-idiotype antibodies using enzyme-linked immunoabsorbent assay with recombinant A20 idiotype protein (Favrille Biotech) as reported previously.25
Immunohistochemistry
Balb/c mice were intramuscularly injected with 6.8 μg cardiotoxin in the quadriceps. Specimens were then collected, cryo-fixed, and stained for hematoxylin and eosin to identify cellular infiltration. The cryo-sections were immunostained for antibodies for monocytes/macrophages (F4/80) and DCs (CD11c). The tissue samples were examined under a 20×/0.40 PH1 objective of a Leica DMLB microscope (Meyer Instruments Inc) equipped with a SPOT RT color camera 2.2.1 (Diagnostic Instruments Inc). Images were captured using SPOT Advanced software 4.7.
Results
Combination therapy with myotoxins potentiated the tumor protection of lymphoma DNA vaccines
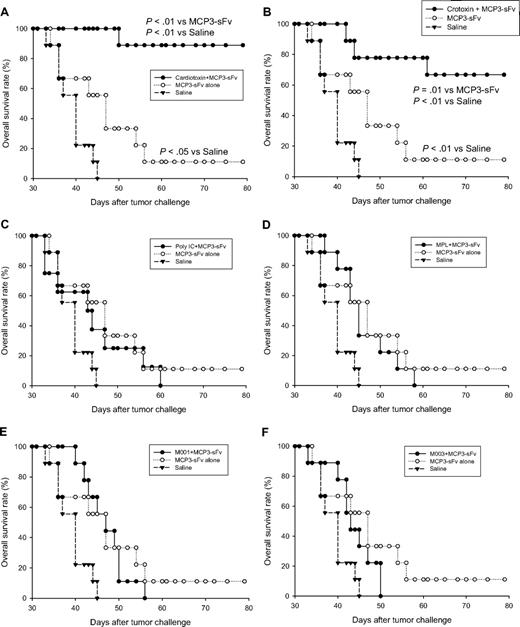
Given that the components involved in tissue inflammation play an important role in triggering immune response, we tested several candidate adjuvants in this category for their potential to improve antitumor effects of a novel DNA vaccine encoding lymphoma idiotype, which was expressed in a single chain format, and genetically fused with monocyte chemotactic protein 3 (MCP3-sFv), as a strategy to target antigen delivery to chemokine receptors on APCs.10 In head-to-head comparisons against conventional adjuvants and TLR agonists, cardiotoxin was the most potent in inducing protective antitumor immunity when combined with the idiotype vaccine. Specifically, syngeneic BALB/c mice that had received 6.8 μg cardiotoxin at DNA vaccine injection sites before administration of the vaccine were highly resistant to tumor challenge (A20 lymphoma, 90%) compared with DNA vaccine alone (10%; P < .01; Figure 1A). These results differed from DNA vaccine combinations with Poly I:C (TLR3 agonist; Figure 1C), MPL (TLR4 agonist; Figure 1D), M001 (TLR7 agonist; Figure 1E), and M003 (TLR7/8 agonist; Figure 1F), respectively, which failed to enhance tumor protection. The potent adjuvant effect of cardiotoxin on protective antitumor immunity was reproduced by crotoxin, another myotoxin with the main component of phospholipase A2 that induces muscle degradation17 (Figure 1B). These results were further confirmed with 2 other chemotactic peptide–fused idiotype DNA vaccines, showing that cardiotoxin significantly enhanced tumor protection when combined with either defensin2β- (Figure 2A) or MIP3α-fused (Figure 2B) antigen. Taken together, these in vivo studies suggest that administering cardiotoxin or crotoxin together with chemokine-fused lymphoma idiotype is an effective strategy to enhance the therapeutic effect of this cancer vaccine.
Administration of myotoxins at vaccination sites significantly enhanced idiotype DNA vaccine-induced tumor protection. Ten BALB/c mice per group were injected intramuscularly with 6.8 μg cardiotoxin (A) or 0.1 μg crotoxin (B) followed by intramuscular vaccination at the same site 5 days later with 50 μg plasmid DNA encoding MCP3 chemokine-fused A20 lymphoma-derived idiotype antigen (MCP3-sFv). TLR agonists, including TLR3 agonist Poly I:C (C), TLR4 agonist MPL (D), TLR7 agonist (M001; E), and TLR7/8 agonist (M003; F), were given, respectively, on the next day of vaccination at a dose of 50 μg.19,20 A total of 3 vaccinations were given with an interval of 14 days. Two weeks after final vaccination, all mice were challenged with a lethal dose of 2 × 105 A20 lymphoma cells by intraperitoneal injection and were followed for survival for 80 days. Control mice were injected with plasmid DNA without candidate adjuvants or with PBS. Survival differences between groups were analyzed by log-rank test. The data shown are from a single experiment, with results presented in multiple panels for clarity.
Administration of myotoxins at vaccination sites significantly enhanced idiotype DNA vaccine-induced tumor protection. Ten BALB/c mice per group were injected intramuscularly with 6.8 μg cardiotoxin (A) or 0.1 μg crotoxin (B) followed by intramuscular vaccination at the same site 5 days later with 50 μg plasmid DNA encoding MCP3 chemokine-fused A20 lymphoma-derived idiotype antigen (MCP3-sFv). TLR agonists, including TLR3 agonist Poly I:C (C), TLR4 agonist MPL (D), TLR7 agonist (M001; E), and TLR7/8 agonist (M003; F), were given, respectively, on the next day of vaccination at a dose of 50 μg.19,20 A total of 3 vaccinations were given with an interval of 14 days. Two weeks after final vaccination, all mice were challenged with a lethal dose of 2 × 105 A20 lymphoma cells by intraperitoneal injection and were followed for survival for 80 days. Control mice were injected with plasmid DNA without candidate adjuvants or with PBS. Survival differences between groups were analyzed by log-rank test. The data shown are from a single experiment, with results presented in multiple panels for clarity.
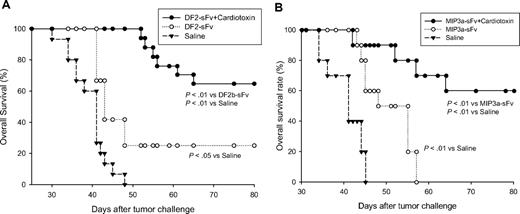
Prophylactic antitumor effects of additional DNA vaccines were significantly improved by the combined vaccination therapy. In prophylactic studies, 10 BALB/c mice per group were immunized with 2 DNA vaccines encoding the idiotype single chain antigen fused to either defensin2β (A) or MIP3α (B), respectively, with or without cardiotoxin, and then challenged with A20 tumor cells as in Figure 1 and followed for survival.
Prophylactic antitumor effects of additional DNA vaccines were significantly improved by the combined vaccination therapy. In prophylactic studies, 10 BALB/c mice per group were immunized with 2 DNA vaccines encoding the idiotype single chain antigen fused to either defensin2β (A) or MIP3α (B), respectively, with or without cardiotoxin, and then challenged with A20 tumor cells as in Figure 1 and followed for survival.
Cardiotoxin-combined vaccination elicited memory antitumor and therapeutic immunity
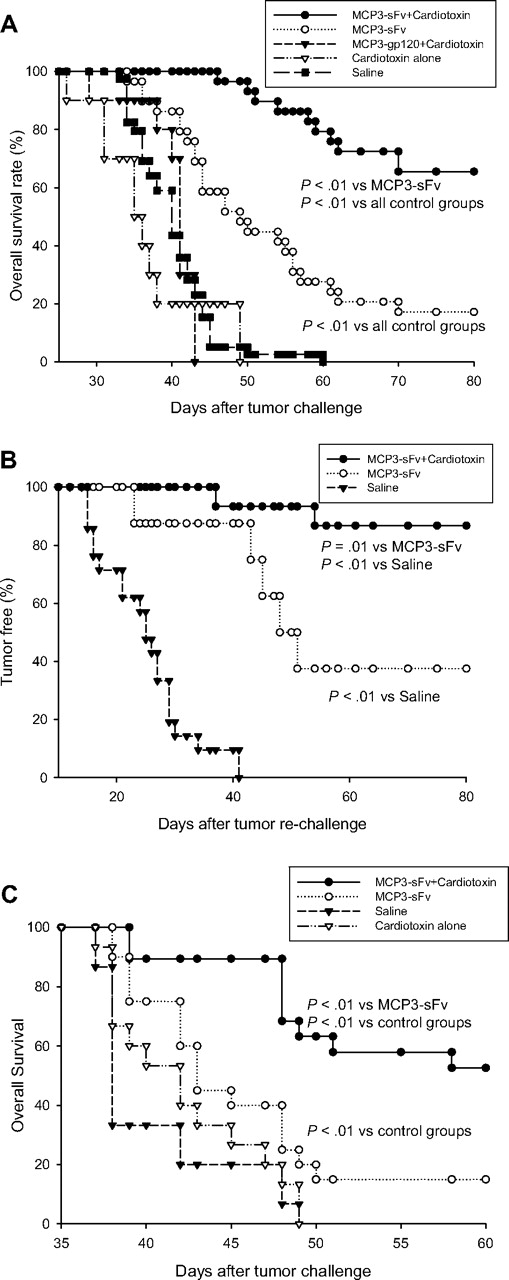
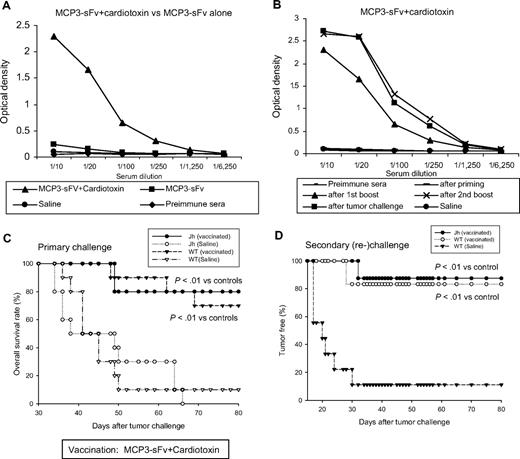
A unique advantage of active immunotherapy is its potential to establish memory immunity to prevent disease relapse; thus, the rational vaccine design should include strategies of inducing memory antitumor response. For this purpose, we examined if the cardiotoxin-combined idiotype vaccine therapy would favor the development of memory immunity against tumor rechallenge. Consistent with the experiment in Figure 1A, mice that received pretreatment of cardiotoxin at vaccination sites were protected from lethal tumor challenge compared with DNA vaccine alone (Figure 3A). Cardiotoxin alone or together with a DNA vaccine encoding MCP3-fused HIV glycoprotein 120, serving as an irrelevant antigen, elicited no protection (Figure 3A). Tumor-free mice surviving this primary challenge were then collected, and, without any further treatment, the mice were rechallenged with the same lethal dose of tumor cells and followed for survival. More than 80% of mice protected by combination DNA vaccine plus cardiotoxin were resistant to tumor rechallenge, compared with less than 40% of mice protected by DNA vaccine alone (P = .01), suggesting immune memory (Figure 3B). Moreover, this combined vaccination strategy also showed its potential to eradicate established tumors in the therapeutic setting, as evident by long-term survival in more than 50% of tumor-bearing mice (Figure 3C).
The combined vaccination therapy elicited memory and therapeutic antitumor immunity. (A) For primary challenge, 10 BALB/c mice per group were vaccinated with 50 μg MCP3-sFv plasmid DNA together with cardiotoxin as in Figure 1. Control mice were injected with plasmid DNA without cardiotoxin pretreatment, with PBS, or with cardiotoxin alone, or with plasmid DNA encoding an irrelevant, HIV gp120 antigen, fused with MCP3. After 3 vaccinations, all mice were challenged with a lethal dose of 2 × 105 A20 lymphoma cells by intraperitoneal injection and followed for survival for 80 days. (B) Tumor-free mice collected from the primary challenge experiments were rechallenged with 2 × 105 A20 tumor cells intraperitoneally and followed for survival. Data represent combined results from 3 independent experiments. Survival differences between groups were analyzed using the log-rank test. (C) In therapeutic studies, 10 BALB/c mice per group were first inoculated with 2 × 105 A20 tumor cells intraperitoneally on day 0. On days 1, 4, 8, and 18 the mice were then vaccinated with plasmid DNA encoding MCP3-fused idiotype sFv with or without cardiotoxin pretreatment 5 days before the first and last vaccination. Control mice were injected with cardiotoxin or PBS alone. Data represent combined results from 2 independent experiments.
The combined vaccination therapy elicited memory and therapeutic antitumor immunity. (A) For primary challenge, 10 BALB/c mice per group were vaccinated with 50 μg MCP3-sFv plasmid DNA together with cardiotoxin as in Figure 1. Control mice were injected with plasmid DNA without cardiotoxin pretreatment, with PBS, or with cardiotoxin alone, or with plasmid DNA encoding an irrelevant, HIV gp120 antigen, fused with MCP3. After 3 vaccinations, all mice were challenged with a lethal dose of 2 × 105 A20 lymphoma cells by intraperitoneal injection and followed for survival for 80 days. (B) Tumor-free mice collected from the primary challenge experiments were rechallenged with 2 × 105 A20 tumor cells intraperitoneally and followed for survival. Data represent combined results from 3 independent experiments. Survival differences between groups were analyzed using the log-rank test. (C) In therapeutic studies, 10 BALB/c mice per group were first inoculated with 2 × 105 A20 tumor cells intraperitoneally on day 0. On days 1, 4, 8, and 18 the mice were then vaccinated with plasmid DNA encoding MCP3-fused idiotype sFv with or without cardiotoxin pretreatment 5 days before the first and last vaccination. Control mice were injected with cardiotoxin or PBS alone. Data represent combined results from 2 independent experiments.
Cellular but not humoral immunity served as the immune mechanism for idiotype DNA vaccine-induced tumor protection
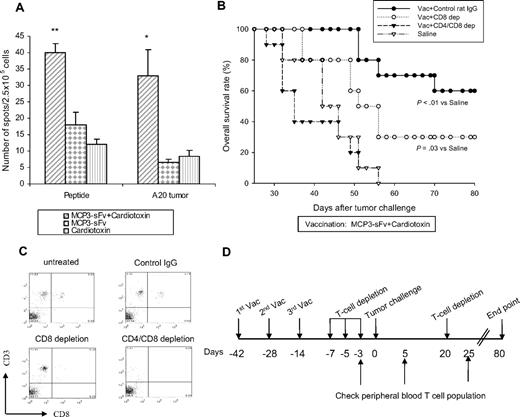
We investigated the relative roles of idiotype-specific T-cell and antibody immune responses in vaccine therapy. First, immunologic studies in vaccinated mice showed the induction of tumor-specific cellular responses (Figure 4A). For example, the mean number of idiotype peptide-specific T cells per 2.5 × 105 splenocytes was 40 (± 2.6) in cardiotoxin-combined mice, compared with 18 (± 3.8) in mice receiving vaccine alone (P < .01) and 33 (± 8) compared with 7 (± 0.9) for tumor-specific T cells, respectively (P < .05). Next, depleting CD8+ T cells in vivo after vaccination plus cardiotoxin was clearly associated with reduced tumor protection, and depletion of both CD4+ and CD8+ T-cell subsets abrogated protection completely (Figure 4B), suggesting a requirement for effector T cells in vaccine-induced antitumor immunity. T-cell depletion was confirmed by showing the absence of CD8+ or CD4+ T cells or both in the peripheral blood (Figure 4C-D).
Combined cardiotoxin and DNA vaccine elicited potent, T cell–dependent, tumor antigen–specific immunity. (A) Splenocytes pooled from 5 BALB/c mice were injected intramuscularly with MCP3-sFv plasmid DNA with or without cardiotoxin as in Figure 1, or cardiotoxin alone. Ten days earlier they were stimulated in vitro with bone marrow–derived DCs pulsed with 5 μg/mL MHC class I–binding A20 idiotype epitope peptide23 for 5 days. The stimulated cells (2 × 105/well) were plated with either the peptide or irradiated A20 tumor cells at a 5:1 T cell/stimulator ratio,24 respectively, and analyzed for INFγ production by ELISPOT after 48 hours. Differences between groups were analyzed using the Student t test (**P < .01; *P < .05). The error bars represent SEM. (B-D) In vivo T-cell depletion was achieved by intraperitoneal injection of 200 μg anti-CD8 mAb (clone 2.43 alone, CD8 depletion) or with 200 μg anti-CD4 mAb (clone GK1.5, CD4/CD8 depletion) according to the schedule in panel D. T-cell depletion was performed on mice vaccinated with cardiotoxin plus MCP3-sFv DNA vaccine as in Figure 1 (10 BALB/c mice per group). Controls received rat IgG instead of T-cell depletion antibodies. After confirming the efficiency of T-cell depletion, which was determined by the presence of CD8 and CD4 T cells in the peripheral blood samples (C), all the mice were challenged with 2 × 105 A20 tumor cells as shown in Figure 1 and followed for survival for 80 days. The data represent 2 independent experiments.
Combined cardiotoxin and DNA vaccine elicited potent, T cell–dependent, tumor antigen–specific immunity. (A) Splenocytes pooled from 5 BALB/c mice were injected intramuscularly with MCP3-sFv plasmid DNA with or without cardiotoxin as in Figure 1, or cardiotoxin alone. Ten days earlier they were stimulated in vitro with bone marrow–derived DCs pulsed with 5 μg/mL MHC class I–binding A20 idiotype epitope peptide23 for 5 days. The stimulated cells (2 × 105/well) were plated with either the peptide or irradiated A20 tumor cells at a 5:1 T cell/stimulator ratio,24 respectively, and analyzed for INFγ production by ELISPOT after 48 hours. Differences between groups were analyzed using the Student t test (**P < .01; *P < .05). The error bars represent SEM. (B-D) In vivo T-cell depletion was achieved by intraperitoneal injection of 200 μg anti-CD8 mAb (clone 2.43 alone, CD8 depletion) or with 200 μg anti-CD4 mAb (clone GK1.5, CD4/CD8 depletion) according to the schedule in panel D. T-cell depletion was performed on mice vaccinated with cardiotoxin plus MCP3-sFv DNA vaccine as in Figure 1 (10 BALB/c mice per group). Controls received rat IgG instead of T-cell depletion antibodies. After confirming the efficiency of T-cell depletion, which was determined by the presence of CD8 and CD4 T cells in the peripheral blood samples (C), all the mice were challenged with 2 × 105 A20 tumor cells as shown in Figure 1 and followed for survival for 80 days. The data represent 2 independent experiments.
Similarly, vaccination also elicited humoral immunity, as shown by serum titers of antigen-specific antibodies that were substantially increased after combining DNA vaccination with cardiotoxin. High levels of anti-idiotype antibodies were maintained even after tumor challenge (Figures 5A-B). However, in contrast with effector T cells, B cells were not required for tumor protection, because DNA vaccine plus cardiotoxin protected both genetically B cell–deficient Jh mice26 and wild-type mice equally from tumor challenge (Figure 5C). More than 80% of tumor-free Jh mice survived from the primary challenge were surprisingly highly resistant to tumor rechallenge, which suggests that anti-idiotype antibodies did not contribute principally to memory antitumor immunity (Figure 5D). The memory antitumor immunity developed in Jh mice was comparable to that found in vaccinated wild-type counterparts (Figure 5D). Together, these data support a critical role for effector T cells activated by cardiotoxin plus idiotype DNA vaccination.
Anti-idiotype antibody response was not required for the vaccine-induced tumor protection. (A-B) Serum samples obtained from mice vaccinated as in Figure 1 were examined for anti-idiotype antibodies by enzyme-linked immunoabsorbent assay with the use of plates coated with recombinant A20 idiotype protein (gift from D. Gold, Favrille Biotech).25 Bound antibodies were detected by HRP-conjugated anti–mouse IgG1. Anti-idiotype antibodies were primarily observed after the first boost dose, peaking after the second boost, and persisting after tumor challenge. (C) Ten wild-type BALB/c (WT) or antibody-deficient Jh mice per group were vaccinated with MCP3-sFv DNA plus cardiotoxin as shown in Figure 1 or with saline and then challenged with tumor and followed for survival. (D) Tumor-free Jh and WT mice from vaccinated groups as in panel C were rechallenged with tumor and followed for survival (Jh, n = 8; WT, n = 7).
Anti-idiotype antibody response was not required for the vaccine-induced tumor protection. (A-B) Serum samples obtained from mice vaccinated as in Figure 1 were examined for anti-idiotype antibodies by enzyme-linked immunoabsorbent assay with the use of plates coated with recombinant A20 idiotype protein (gift from D. Gold, Favrille Biotech).25 Bound antibodies were detected by HRP-conjugated anti–mouse IgG1. Anti-idiotype antibodies were primarily observed after the first boost dose, peaking after the second boost, and persisting after tumor challenge. (C) Ten wild-type BALB/c (WT) or antibody-deficient Jh mice per group were vaccinated with MCP3-sFv DNA plus cardiotoxin as shown in Figure 1 or with saline and then challenged with tumor and followed for survival. (D) Tumor-free Jh and WT mice from vaccinated groups as in panel C were rechallenged with tumor and followed for survival (Jh, n = 8; WT, n = 7).
Myotoxins induced sterile inflammation and recruitment of APCs
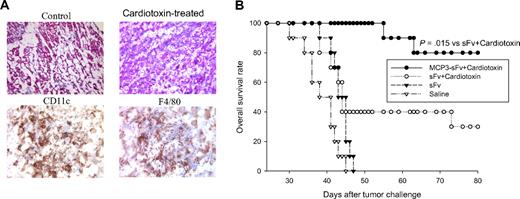
To further elucidate the unexpected immunologic effects of cardiotoxin, we examined muscles at cardiotoxin injection sites histologically and observed marked cellular infiltration (Figure 6A). Further identification showed the majority of infiltrating cells were DCs and monocytes/macrophages (Figure 6A).
Cardiotoxin administration recruits antigen-presenting cells and converts nonimmunogenic, unfused antigen into a protective vaccine. (A) Cardiotoxin alone-treated (6.8 μg by intramuscular injection) quadriceps were collected, cryo-fixed, sectioned, and stained for hematoxylin and eosin. Further identification of infiltrated cells was performed by immunostaining tissue sections with cell-specific markers for DCs (CD11c) and monocytes/macrophages (F4/80; representative of 6 quadriceps analyzed). (B) Ten syngeneic BALB/c mice per group were immunized with DNA vaccines encoding chemokine-fused (MCP3-sFv) or free antigen (sFv) plus cardiotoxin and challenged with lethal tumor as in Figure 1. The data are representative of 2 identical experiments.
Cardiotoxin administration recruits antigen-presenting cells and converts nonimmunogenic, unfused antigen into a protective vaccine. (A) Cardiotoxin alone-treated (6.8 μg by intramuscular injection) quadriceps were collected, cryo-fixed, sectioned, and stained for hematoxylin and eosin. Further identification of infiltrated cells was performed by immunostaining tissue sections with cell-specific markers for DCs (CD11c) and monocytes/macrophages (F4/80; representative of 6 quadriceps analyzed). (B) Ten syngeneic BALB/c mice per group were immunized with DNA vaccines encoding chemokine-fused (MCP3-sFv) or free antigen (sFv) plus cardiotoxin and challenged with lethal tumor as in Figure 1. The data are representative of 2 identical experiments.
We hypothesized that the recruitment of APCs into vaccination sites by myotoxins might potentiate the action of the chemokine motif in the fusion vaccine to enhance vaccine-triggered antitumor immunity. This hypothesis was tested by showing that, even though cardiotoxin administration was able to convert nonimmunogenic, unfused antigen into an effective immunogen that induced antitumor immunity (Figure 6B), the optimal adjuvant effect of cardiotoxin was only observed with antigen fused to chemokine, which probably targets DCs for a more efficient antigen delivery, as shown by our previous experiments that mixing free antigen and chemokine was not sufficient to induce immunity.10
Discussion
Although non-Hodgkin lymphoma is responsive to chemotherapy and remission is common, most patients eventually relapse. Therefore, novel, streamlined, potent therapeutic approaches are required. Lymphoma idiotype vaccine is a promising approach for eradication of minimal disease. In combination with chemotherapy, its potential to prevent relapses and to prolong disease-free survival has been supported by clinical studies.4,5,8,27 DNA vaccines represent a second-generation vaccine approach with the aim of simplifying the manufacturing process and improving the potency of antitumor immunity. The latter can be achieved by genetic modification of idiotype antigen for targeted delivery to APCs.9,10 For example, our previous studies showed that genetic fusion of lymphoma idiotype in single chain format with chemokines enhanced the immunogenicity of the antigen and induced tumor protection in lymphoma mouse models.10,21
However, most controlled trials of cancer vaccines, when used as monotherapy, have failed. It is clear that successful therapeutic vaccination will require combination strategies, either with additional proinflammatory agents that can trigger the induction of specific immune responses or by agents that provide release from immune suppression at the effector phase of the immune response. In our studies, when combined with idiotype DNA vaccines, myotoxins elicited potent antitumor immunity. Importantly, a desirable memory T-cell response was also elicited by these vaccine and cardiotoxin combinations. Myotoxins have been used previously to induce muscle regeneration and to enhance uptake of DNA vaccines, with limited success.18 However, our data suggest an additional effect of cardiotoxin and crotoxin inducing sterile inflammation (Figure 6A). Future mechanistic studies are warranted to fully elucidate this previously undescribed immune effect on the microenvironment.
The potency of this combined vaccine therapy was also evident by the ability of cardiotoxin to convert an otherwise non–immunogenic antigen (sFv alone; Figure 6B) into a protective immunogen. This feature is highly desirable in designing cancer vaccines because most candidate tumor-associated antigens are self-proteins. The therapeutic efficacy of cancer vaccine is largely determined by its potential to break immune tolerance to these self-antigens. Thus, myotoxins may represent a new class of adjuvants that may be generally applied to the development of active immunotherapy against other types of malignancies.
Adaptive immunity includes cellular and humoral responses. As shown in current studies, both idiotype-specific T cells and antibodies were induced by combining idiotype DNA vaccines with myotoxins. However, the exact roles of effector T cells and antibodies in vaccine-induced eradication of tumor cells are not fully elucidated. Antibodies have generally been thought to be the primary cellular mechanism underlying the antitumor effects of vaccines against lymphoma idiotypes. Serum level of anti-idiotype antibodies has been frequently used for monitoring the efficacy of vaccination in patients. Using antibody-deficient Jh mice, for the first time, we have shown that the antibody response was not required for idiotype-induced antitumor effects, even though the combination of vaccines with cardiotoxin strikingly enhanced serum anti-idiotype antibody titers. Prior studies in a different mouse lymphoma model, in which depletion of effector T cells in vivo abrogated tumor protection, also suggested a role for T-cell immunity.10 This novel finding suggests a focus on induction of T-cell immunity in designing future idiotype-based immunotherapy strategies. The significance of enhancing T-cell responses is apparent with the widespread use of rituximab in the treatment of B-cell NHLs, because of impaired humoral immunity as the result of B-cell depletion by rituximab.
Optimal antitumor immunity induced by cardiotoxin combinations required fusion vaccines that targeted receptors on DCs for antigen delivery, exemplified by genetic fusion of antigen to chemokine receptor ligands (Figure 6B). Our working hypothesis is that chemokine motif in the de novo–synthesized fusion proteins facilitates APCs to capture antigens via chemokine receptor-mediated endocytosis, and subsequently triggers antigen “cross-presentation,” loading antigens to MHC class I pathway for T-cell priming. This principle was shown in studies with a MIP3a-fused melanoma antigen gp100 protein, which when pulsed onto DCs, activated the antigen-specific CD8+ T cells, and this effect was abrogated by blockage of the MHC class I pathway.28 We have previously shown that chemokine receptor ligands fused to antigen, such as defensin2β, can also function to induce DC maturation.22 Using these data collectively with our current results, we conclude that the ideal, fully optimized vaccine strategies should simultaneously create a favorable immune microenvironment to recruit and activate APCs and to target antigen delivery to APCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Leukemia & Lymphoma Society (6003-07 and 7262-08), Department of Defense (W81XWH-07-1-0345; L.W.K.), and the Lymphoma Research Foundation (H.Q.).
Authorship
Contribution: H.Q., S.-c.C., and S.S.N. designed and performed the studies, conducted data analysis, and prepared the manuscript; Y.L. and J.W. conducted animal experiments; and L.W.K. developed the project, supervised the research, and wrote the manuscript together with Y.-J.L.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Larry W. Kwak, Department of Lymphoma and Myeloma, Center for Cancer Immunology Research, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: lkwak@mdanderson.org.
References
Author notes
H.Q. and S.-c.C. contributed equally to this study

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal