Abstract
Forkhead box P3 (FOXP3) is constitutively expressed by CD4+CD25hi regulatory T cells (nTregs). Mutations of FOXP3 cause a severe autoimmune syndrome known as immune dysregulation polyendocrinopathy enteropathy X-linked, in which nTregs are absent or dysfunctional. Whether FOXP3 is essential for both differentiation and function of human nTreg cells remains to be demonstrated. Because FOXP3 is an X-linked gene subject to X-chromosome inactivation (XCI), we studied 9 healthy female carriers of FOXP3 mutations to investigate the role of wild-type (WT) versus mutated FOXP3 in different cell subsets. Analysis of active WT versus mutated (mut)–FOXP3 allele distribution revealed a random pattern of XCI in peripheral blood lymphocytes and in naive and memory CD4+T cells, whereas nTregs expressed only the active WT-FOXP3. These data demonstrate that expression of WT-FOXP3 is indispensable for the presence of a normal nTreg compartment and suggest that FOXP3 is not necessary for effector T-cell differentiation in humans.
Introduction
Naturally occurring CD4+CD25hi regulatory T cells (nTregs) play a central role in immune tolerance. nTregs are generated in the thymus, comprise 5% to 10% of peripheral CD4+ T cells, and constitutively express forkhead box P3 (FOXP3), a transcription factor essential for their regulatory activity.1 Indeed, knockdown of FOXP3 in nTregs reduces suppressive capacity and overexpression of FOXP3 confers suppressive activity to conventional CD4+ T cells.2-4 However, an essential role of FOXP3 in the development and maintenance of nTregs in humans has not yet been shown. Moreover, FOXP3 can be expressed in activated effector T cells, suggesting that it may also be important for cells other than nTregs.5,6
In male infants, mutations of FOXP3 cause a severe autoimmune disease known as immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX).7-9 Depending on the mutation, nTregs may be present in IPEX patients, but display an impaired function.10
The FOXP3 gene (Xp11.23) is subject to X-chromosome inactivation (XCI),11 a random process by which 1 of the 2 X-chromosomes is methylated during embryogenesis and remains stably inactive in daughter cells. Accordingly, female carriers of FOXP3 mutations are expected to be cell mosaics in which either the wild-type (WT) or the mutated (mut)–FOXP3 allele is active. Therefore, they represent a unique system to investigate the role of WT versus mut-FOXP3 in nTregs and other cell subsets in humans. Because in heterozygous Foxp3wt/null female mice, only nTregs expressing WT-Foxp3 have the nTreg phenotype and are maintained in the periphery,12 we investigated whether expression of human WT-FOXP3 is necessary for the development and/or homeostasis of nTregs, by analyzing the pattern of XCI in T-cell subsets from women carrying FOXP3 mutations.
Methods
Subjects
Carriers of FOXP3 mutations are healthy adults with no autoimmune symptoms. Peripheral blood was obtained upon informed consent from healthy females and carriers of FOXP3 mutations in accordance with approval from the Internal Ethical Committee of the San Raffaele Scientific Institute (protocol TIGET02) and with the Declaration of Helsinki.
For complete materials and methods information, see supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
Healthy carriers of FOXP3 mutations are mosaics of cells expressing either the WT or the mut-FOXP3 allele
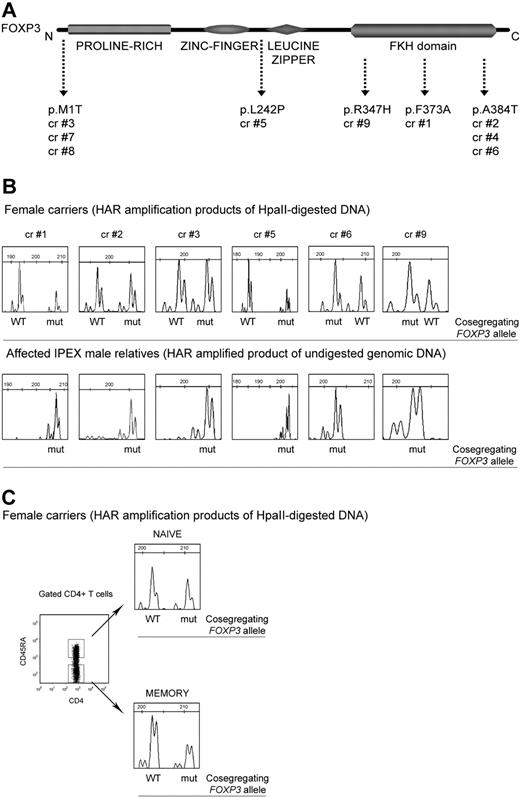
Nine healthy female carriers of 5 different FOXP3 mutations, relatives of severely affected IPEX patients, were studied (Figure 1A and supplemental methods). The distribution of active WT and mut-FOXP3 alleles in peripheral blood mononuclear cells (PBMCs; n = 9), CD4+ T (n = 8), CD8+ T (n = 3), CD56+ natural killer (n = 2), and CD19+ B (n = 3) cells was evaluated by determining the pattern of XCI11,13 and by correlating it with the presence of the active mut-FOXP3 allele. To this end, we analyzed the polymorphic CAG repeats of the HAR gene to distinguish the 2 X-chromosomes in each carrier and we used them to track the segregation of the mut-FOXP3 allele inherited by the relevant IPEX patient. The HAR and the FOXP3 genes are located 10-cM apart, that is, there is a 10% chance of recombination between them. Although caution should be used in assuming that segregation analysis at the HAR locus may provide information on the active WT or mut-FOXP3 allele in IPEX families, we did not observe any recombination in the families studied here (not shown).
The pattern of XCI in PBMCs and CD4+ T-cell subsets derived from carriers of FOXP3 mutations is random. (A) Carriers 1, 2, 4, 6, and 9 have mutations in the forkhead/winged-helix (FKH) DNA-binding domain, whereas carrier 5 has a mutation in exon 6 between the C2H2 zinc finger and the leucine zipper domains. Carriers 3, 7, and 8 have a nucleotide substitution within the ATG translation start site, which completely abrogates the expression of the FOXP3 protein (FOXP3-null mutation; S.D.N., unpublished data, November 7, 2008). Carriers 2 and 4 and carriers 3, 7, and 8 are members of the same families. (B) Pherograms of XCI analyses of total PBMCs derived from carriers of FOXP3 mutations are shown (top panel). For carriers who are members of the same family (carriers 2 and 4, carriers 3, 7, and 8) only the pherogram of PBMCs derived from 1 representative subject is shown. CAG repeats of the HAR gene were amplified after digestion with the methylation-sensitive enzyme HpaII so that only methylated (inactive) sequences were amplified. For each HAR allele, the cosegregating WT or mut-FOXP3 allele is indicated according to CAG repeats that cosegregate with the mut-FOXP3 in the relevant IPEX patients (bottom panel). Genomic DNA derived from PBMCs of IPEX patients was not digested because in males the unique X-chromosome is unmethylated and active. (C) XCI was analyzed in CD4+ naive and memory T cells sorted according to their expression of CD45RA. The pherograms of CD4+ T-cell populations of the representative carrier 4 are shown. cr indicates carrier.
The pattern of XCI in PBMCs and CD4+ T-cell subsets derived from carriers of FOXP3 mutations is random. (A) Carriers 1, 2, 4, 6, and 9 have mutations in the forkhead/winged-helix (FKH) DNA-binding domain, whereas carrier 5 has a mutation in exon 6 between the C2H2 zinc finger and the leucine zipper domains. Carriers 3, 7, and 8 have a nucleotide substitution within the ATG translation start site, which completely abrogates the expression of the FOXP3 protein (FOXP3-null mutation; S.D.N., unpublished data, November 7, 2008). Carriers 2 and 4 and carriers 3, 7, and 8 are members of the same families. (B) Pherograms of XCI analyses of total PBMCs derived from carriers of FOXP3 mutations are shown (top panel). For carriers who are members of the same family (carriers 2 and 4, carriers 3, 7, and 8) only the pherogram of PBMCs derived from 1 representative subject is shown. CAG repeats of the HAR gene were amplified after digestion with the methylation-sensitive enzyme HpaII so that only methylated (inactive) sequences were amplified. For each HAR allele, the cosegregating WT or mut-FOXP3 allele is indicated according to CAG repeats that cosegregate with the mut-FOXP3 in the relevant IPEX patients (bottom panel). Genomic DNA derived from PBMCs of IPEX patients was not digested because in males the unique X-chromosome is unmethylated and active. (C) XCI was analyzed in CD4+ naive and memory T cells sorted according to their expression of CD45RA. The pherograms of CD4+ T-cell populations of the representative carrier 4 are shown. cr indicates carrier.
The pherograms of the XCI analysis performed on DNA from PBMCs are shown in Figure 1B. For each mutation, the pherogram of amplified nondigested DNA of the relevant IPEX patient PBMCs identifies the mut-FOXP3 allele. In all of the carriers' PBMCs, the distribution of active WT-FOXP3 and mut-FOXP3 is random. The same results were obtained in all of the analyzed subpopulations (not shown). The partial skewing toward the mutated allele in carriers 1 and 5 is considered physiologic, because, in one-third of healthy females, one active X-chromosome is predominant (≥ 75%) in hematopoietic cells.14
These data indicate that, independently of the mutation, carriers are cell mosaics in which either the wild-type or the mut-FOXP3 allele is present on the active X-chromosome. In addition, we found that the distribution of XCI in naive CD4+CD45RA+ T (n = 4) and memory CD4+CD45RA− T (n = 3) cells was random (Figure 1C). The coexistence of WT and mut-FOXP3 cells indicates that FOXP3 mutations do not interfere with in vivo differentiation of CD4+ T cells into memory cells.
Carriers of FOXP3 mutations have functionally normal nTregs that exclusively express the WT-FOXP3 allele
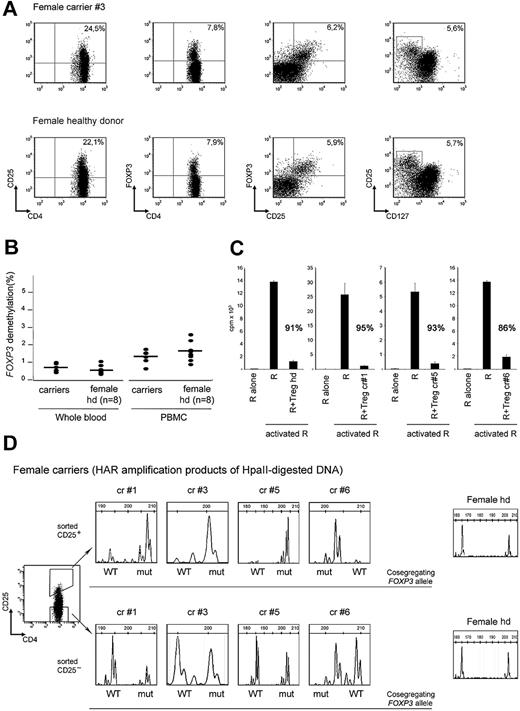
All carriers had normal proportions of nTregs coexpressing CD25, FOXP3, and CD127low/− (Figure 2A and supplemental Table 1). CD4+CD25+ T cells represented 21% (± 4%; mean ± SD, n = 8); CD4+FOXP3+, 6% (± 1%; n = 7); CD25+FOXP3+, 5% (± 2%; n = 6); and CD25+CD127low, 5% (± 1%; n = 6) of the CD4+T cells, and were in the range of those of healthy female donors (n = 12). The percentage of demethylated copies of the FOXP3 Treg-specific demethylated region (TSDR) was used to quantify nTregs.15,16 In DNA extracted from carriers' PBMCs or whole blood (n = 7) the percentages of demethylated TSDR were 0.7 (± 0.3) and 1.3 (± 0.4), respectively, and were comparable with that of female controls (n = 8), 0.6 (± 0.2) and 1.6 (± 0.6; P > .05; Figure 2B). The capability of CD4+CD25hi T cells derived from carriers of FOXP3 mutations (n = 3) to suppress the proliferation of allogeneic responder T cells was comparable with that of healthy donors (Figure 2C).
Carriers of FOXP3 mutations have functionally normal nTregs with exclusive expression of the WT-FOXP3 allele. (A) Expression of FOXP3, CD25, and CD127 in CD4+ gated PBMCs derived from the representative carrier 3 (top panel) and a representative healthy female donor (bottom panel). (B) FOXP3 TSDR demethylation levels in genomic DNA samples from whole blood and total PBMCs derived from carriers of FOXP3 mutations and healthy donor females (n = 8). (C) The ability of freshly isolated CD4+CD25+ T cells (Treg) of carriers 1, 5, and 6 and of a representative healthy donor to suppress activated responder CD4+CD45RO+ effector T cells (R) is shown. Percentages indicate the inhibition of proliferation. Error bars indicate SD. (D) The pherograms of XCI analyses of sorted CD4+CD25hi (top panel) and CD4+CD25− (bottom panel) T cells derived from carriers 1, 3, 5, and 6 (n = 4) and from 1 representative healthy female donor (n = 3) are shown. In CD4+CD25hi T cells derived from carriers of FOXP3 mutations, only the inactive repeats that cosegregate with the mut-FOXP3 allele can be amplified, indicating that only the WT-FOXP3 allele is active. In contrast, in CD4+CD25hi T cells derived from healthy donors (n = 3) both the chromosomes are active. In sorted CD4+CD25− T cells derived from carriers of FOXP3 mutations (n = 4) and healthy female donors (n = 3), CAG repeats of both chromosomes can be amplified, indicating that both the WT and the mut alleles are active. For each repeat the cosegregating WT and mut-FOXP3 alleles are indicated. cr indicates carrier; hd, healthy donor.
Carriers of FOXP3 mutations have functionally normal nTregs with exclusive expression of the WT-FOXP3 allele. (A) Expression of FOXP3, CD25, and CD127 in CD4+ gated PBMCs derived from the representative carrier 3 (top panel) and a representative healthy female donor (bottom panel). (B) FOXP3 TSDR demethylation levels in genomic DNA samples from whole blood and total PBMCs derived from carriers of FOXP3 mutations and healthy donor females (n = 8). (C) The ability of freshly isolated CD4+CD25+ T cells (Treg) of carriers 1, 5, and 6 and of a representative healthy donor to suppress activated responder CD4+CD45RO+ effector T cells (R) is shown. Percentages indicate the inhibition of proliferation. Error bars indicate SD. (D) The pherograms of XCI analyses of sorted CD4+CD25hi (top panel) and CD4+CD25− (bottom panel) T cells derived from carriers 1, 3, 5, and 6 (n = 4) and from 1 representative healthy female donor (n = 3) are shown. In CD4+CD25hi T cells derived from carriers of FOXP3 mutations, only the inactive repeats that cosegregate with the mut-FOXP3 allele can be amplified, indicating that only the WT-FOXP3 allele is active. In contrast, in CD4+CD25hi T cells derived from healthy donors (n = 3) both the chromosomes are active. In sorted CD4+CD25− T cells derived from carriers of FOXP3 mutations (n = 4) and healthy female donors (n = 3), CAG repeats of both chromosomes can be amplified, indicating that both the WT and the mut alleles are active. For each repeat the cosegregating WT and mut-FOXP3 alleles are indicated. cr indicates carrier; hd, healthy donor.
To determine the pattern of XCI in nTregs, CD4+CD25hi T cells were sorted from 4 carriers of mutations affecting different regions of FOXP3. In CD4+CD25hi T cells from all the carriers analyzed, only the X-chromosome carrying the WT-FOXP3 allele was active (Figure 2D). In contrast, either the WT-FOXP3 or the mut-FOXP3 allele was active in CD4+CD25− T cells of the same carriers. Sorted CD4+CD25hi and CD4+CD25− T cells from 3 healthy females displayed a random pattern of XCI, further confirming that in carriers of FOXP3 mutations only nTregs bearing the active WT-FOXP3 allele are selected to give rise to a normal nTreg subset.
In conclusion, we found a random XCI in PBMCs and CD4+ effector T cells of female carriers of FOXP3 mutations, but not in their nTregs, which expressed only the WT-FOXP3. These data represent the first evidence that FOXP3 is necessary for the development of normal nTreg populations in humans. Although our study does not clarify whether WT-FOXP3–expressing nTregs are already selected in these subjects during thymic differentiation, it provides evidence of a clear advantage for these cells in the periphery, suggesting that only nTregs with WT-FOXP3 survive in healthy subjects to maintain immune homeostasis. This hypothesis is in accordance with the demonstration that in Foxp3ΔGFP mice, lacking the Foxp3 protein, Foxp3 is not necessary for thymic development of nTregs, but is indispensable to maintain a functionally normal peripheral nTreg population.17 Furthermore, the observation that nTregs are present in PBMCs of IPEX patients10 demonstrates that mut-FOXP3 does not prevent the emergence of nTregs from the thymus, indirectly reinforcing the hypothesis of a peripheral selection of WT-FOXP3 nTregs in carriers. Interestingly, long-term survival of donor-derived nTregs, despite the loss of donor peripheral chimerism in other cell populations, has been demonstrated in an IPEX patient who successfully underwent bone marrow transplantation,18 further supporting the evidence that nTregs expressing the WT-FOXP3 allele have a selective advantage.
Altogether, the observations that WT-FOXP3 nTregs can maintain immune homeostasis in healthy subjects, despite the presence of other cell subsets expressing mut-FOXP3, support the rationale for cell and gene therapy–based approaches to restore nTreg function in IPEX patients. These treatments would represent a valid alternative to immunosuppressive therapy, only partially efficacious, and to bone marrow transplantation, which can be beneficial but is limited by toxicity and availability of human leukocyte antigen–compatible donors.9,19
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We deeply appreciate the support and cooperation of all the carriers of FOXP3 mutations.
This work was supported by a grant from the Italian Telethon Foundation, Rome (GGP07241; R.B.) and a grant from Cariplo-Nobel (M.G.R.).
Authorship
Contribution: S.D.N. performed research and wrote the paper; M.C., L. Passerini, A.N.M., U.B., I.T., S.O., and L. Perroni performed research; S.V., E.V., A.T., A.J., and G.C. provided clinical information and samples from carriers; M.K.L. and M.G.R. revised data and the paper; and R.B. designed and coordinated the research and wrote the paper.
Conflict-of-interest-disclosure: U.B. and S.O. are employees/owners of Epiontis GmbH, Berlin, Germany. The remaining authors declare no competing financial interests.
Correspondence: Rosa Bacchetta, San Raffaele Telethon Institute for Gene Therapy (HSR-TIGET), via Olgettina, 58-20132 Milano, Italy; e-mail: rosa.bacchetta@hsr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal