Abstract
Severe sepsis is one of the leading causes of death worldwide. High mortality rates in sepsis are frequently associated with neutropenia. Despite the central role of neutrophils in innate immunity, the mechanisms causing neutropenia during sepsis remain elusive. Here, we show that neutropenia is caused in part by apoptosis and is sustained by a block of hematopoietic stem cell (HSC) differentiation. Using a sepsis murine model, we found that the human opportunistic bacterial pathogen Pseudomonas aeruginosa caused neutrophil depletion and expansion of the HSC pool in the bone marrow. “Septic” HSCs were significantly impaired in competitive repopulation assays and defective in generating common myeloid progenitors and granulocyte-monocyte progenitors, resulting in lower rates of myeloid differentiation in vitro and in vivo. Delayed myeloid-neutrophil differentiation was further mapped using a lysozyme–green fluorescent protein (GFP) reporter mouse. Pseudomonas's lipopolysaccharide was necessary and sufficient to induce myelosuppresion and required intact TLR4 signaling. Our results establish a previously unrecognized link between HSC regulation and host response in severe sepsis and demonstrate a novel role for TLR4.
Introduction
Sepsis is a complex clinical syndrome, a devastating consequence of bacterial infection that frequently causes severe organ dysfunction, and is the leading cause of death in noncoronary intensive care units.1 The severe complications present during sepsis are largely due to the excessive release of cytokines that lead progressively to endothelial dysfunction, coagulation cascade activation, microvascular injury, and, in many cases, multiple organ failure.1 The systemic inflammation underlying sepsis results initially from the “failure” of the host innate immune system to control invasive pathogens. A key component of the host innate response to bacterial pathogens is the neutrophil.2 During infection, neutrophils rapidly migrate to the site of inflammation where they initiate their antimicrobial activity. Due to their short life span, neutrophils have to be supplied continuously during infection by expansion of myeloid progenitors in the bone marrow (BM).3 Thus, the ability of the BM to respond to infections by expanding the progenitors and producing differentiated cells capable of destroying the microbial pathogens, while preserving an intact stem cell pool, is a critical feature of host defense that translates into the difference between resolving an infection or succumbing to it. Despite the central role of neutrophils in innate immunity, the mechanisms of neutropenia and myelosuppression during sepsis remain elusive.
The use of steady-state hematopoiesis mouse models and bone marrow transplantation–induced stress have permitted great progress in identifying key molecules that coordinate hematopoietic stem cell (HSC) self-renewal, proliferation, and differentiation during normal adult hematopoiesis.4,5 However, the in vivo mechanisms by which the HSC compartment responds to sepsis is poorly understood. Severe sepsis is one of the most dramatic examples of inadequate host response to inflammation, where an initial hyperreactive response is often followed by profound neutropenia and “immune paralysis.”6 However, the precise dynamic of HSC response and the cause of neutropenia during sepsis have not been investigated.
Previous studies had shown that Pseudomonas aeruginosa can cause profound neutropenia in burns. Using a burn mouse model7,8 that closely recapitulates the lethal sepsis occurring in patients with overwhelming Gram-negative bacterial infection, we have mapped the cellular dynamics that are altered in the BM compartment during sepsis. We observed that P aeruginosa induced a dramatic expansion of the stem cell compartment associated with failure to regenerate neutrophils. These effects were not induced by a nonlethal isogenic P aeruginosa mutant associated with high survival and defective in lipopolysaccharide (LPS) production, but were recapitulated by injection of purified LPS. In addition, we show that TLR4 signaling, triggered by LPS, directs a dysfunctional expansion of HSCs in response to bacterial infection, resulting in low engraftment potential, both in the long and short terms, and in a defective ability to generate myeloid progenitors.
Methods
Mice
Bacteremia and endotoxemia models were performed in several mouse strains: CD1 and C57BL/6J mice (The Jackson Laboratory); FVB/N-TgN Lys-GFP reporter mice (Dr Graf laboratory, Center for Genomic Regulation, Barcelona, Spain);9 C3H/HeJ and C3H/OuJ mice (The Jackson Laboratory). Sex-matched mice of both sexes between the ages of 8 and 12 weeks were used. B6.SJL-PtrcaPep3b/BoyJ (BoyJ; CD45.1) mice (In vivo Therapeutics Core, Wells Center, Indiana University) were used as recipient for transplants. All animal studies were reviewed and approved by the Institutional Animal Care of the Massachusetts General Hospital and by the Indiana University Laboratory Animal Research Center Committee on Animal Research.
Bacteremia and endotoxemia models
A mouse full-thickness skin burn model was used.7,8 Briefly, mice were subjected to a 7% scalding injury on the abdominal surface, and the bacterial inoculum was delivered subcutaneously under the scald eschar. Independent sets of mice were infected with bacterial inoculums of PA14 or 33C7 strains (∼ 2-3 × 105 CFU/mL). Mice that received scalding injury only and healthy mice were used as controls. Blood samples from challenged animals were collected and dilutions were plated to assess the number of bacteria present at different time points.
Mice were subjected to intraperitoneal inoculation of 0.8 mg/kg (approximately 20 μg/mouse) of P aeruginosa LPS purchased from Sigma (L 8643).
Bone marrow transplantation
Competitive repopulation assay (CRA) was used to evaluate the repopulation ability of lineage-negative (Lin−) or LSK cells sorted from controls, septic, or LPS-challenged animals. Recipients (C57BL/6-CD45.1, female; The Jackson Laboratory) were irradiated with a split dose of 10 Gy 16 hours before transplantation. Lin− or LSK donor cells were obtained from 8- to 12-week-old female C57BL/6-CD45.2 mice (The Jackson Laboratory). Competitive BM cells were prepared as a single-cell suspension from Boy/J CD45.1 mice and admixed with CD45.2 test cells. Engraftment was evaluated at 4-week intervals by collecting the peripheral blood (PB) for analysis of CD45.2 and lineage markers.
Reagents
Recombinant mouse stem cell factor (SCF), interleukin-3 (IL-3), and granulocyte colony-stimulating factor (G-CSF) were purchased from R&D Systems.
Morphology and histologic analysis
BM smears from tibias and cytospins (105 cells) were stained with Wright-Giemsa using the Hema-Tek system. Tissues were fixed in 10% neutral-buffered formalin, paraffin embedded, sectioned at 5 μm, and mounted for staining with hematoxylin and eosin.
Flow cytometry and identification of hematopoietic stem and progenitor cells
BM cells were incubated with a lineage antibody cocktail (anti–Mac-1, anti-Gr1, anti-B220, anti-Ter119, anti-CD3, anti-CD8, and anti-CD4) followed by negative selection by magnetic-activated cell sorter (MACS) separation system (Miltenyi Biotec) or sorting by fluorescent-activated cell sorter (FACS). Lineage-depleted cells were stained with anti–c-Kit, anti-Sca1, and anti–IL-7R and sorted. Identification of defined subsets was conducted as follows: HSC (Lin− IL-7Rα− CD34− c-Kit+, Sca1+), common myeloid progenitor (CMP; Lin− IL-7Rα− c-Kit+, Sca1− CD34+ FcγRII/IIIlo), granulocyte-monocyte progenitor (GMP; Lin− IL-7Rα− c-Kit+, Sca1− CD34+ FcγRII/IIIhi), megakaryocytic-erythroid precursor (MEP; Lin− IL-7Rα− c-Kit+, Sca1− CD34− FcγRII/IIIlo), and common lymphoid progenitors (CLP; Lin− IL-7Rα+ c-Kitlo, Sca1lo). Staining includes the following antibodies: FITC-conjugated CD3 (17A2), CD4 (RM4-5), CD8 (53-6.7), B220 (RA3-6B2), Ter119, Mac1 (M1/70), Gr1 (RB6-8C5); PECy7-IL7Rα (A7R34), PECy5.5-Sca1, Alexa 750–c-Kit (2B8), APC-FcRγII/III93, Pacific blue–CD34 (RAM34), PE-conjugated anti-TLR4–MD2 Complex (MTS510). PB was labeled with anti-CD45.1–FITC and anti-CD45.2–APC plus CD3-PE, CD4-PE, B220-PE, CD8-PE Gr-1–PE, Mac1-PE, or F4/80-PE. Sca1-FITC (E13-161.7), Gr1/Mac1/F4-80–PE, and c-Kit–APC (2B8) were used in in vitro differentiation. LSRII, FACSCalibur, and FACSAria (BD Biosciences) were used for analysis and sorting. Data were analyzed with FlowJo (TreeStar) and CellQuest software. For analysis of rare populations, 5 × 105 to 1 × 106 events were collected.
Serum-free cell cultures
BM cells from control or PA14- or LPS-challenged mice were harvested from femurs by flushing with phosphate-buffered saline (PBS)/0.2mM EDTA. Cells were lineage depleted using the lineage cell depletion kit from Miltenyi Biotec or sorted for the LSK subset. Cells were grown in X-VIVO-15 media (Lonza BioWhittaker) supplemented with SCF (50 ng/mL; R&D Systems), IL-3 (20 ng/mL; R&D Systems) and G-CSF (10 ng/mL), seeded at 5 × 105 cells/mL in 48- or 96-well plates, and cultured at 37°C, 5% CO2 for up to 7 days.
Cell-cycle and apoptosis analysis
BM fraction enriched in Lin− cells by immunomagnetic separation was labeled with antibodies detecting the LSK population and by Hoechst and propidium iodide (PI) as described10 and analyzed by FACS. In independent experiments, Lin− cells immediately after harvest and purification were cultured in X-VIVO-15 media supplemented with BrdU (10 μM), and no growth factors, for a 2-hour pulse. Cells were collected and cell-cycle analysis was performed using the BrdU-APC Flow kit (BD Pharmingen). Cell death was measured by labeling BM cells with annexin V and PI following the manufacturer's instructions, in combination with markers for LSK, common progenitors, Gr1, and Mac1. Samples were analyzed by FACS.
Long-term culture with limiting dilutions and colony assays
Cobblestone area–forming cell (CAFC) assay11 was used to quantify primitive cells within the BM. Lin− cells from PA14- or LPS-challenged mice and controls were seeded in limiting dilution over feeder layers of murine BM stroma cultured for 5 weeks and measured as described.10 BM cells from control and LPS-challenged mice were seeded in methylcellulose at 10 000/mL and evaluated for colony-forming ability at days 7 through 10.
Quantitative qRT-PCR analysis of gene expression
Total RNA was isolated using the RNAeasy kit (QIAGEN). DNAse (QIAGEN)–treated RNA was used to generate cDNAs by reverse transcription according to the manufacturer's instructions (SuperScript II kit; Invitrogen). Polymerase chain reactions (PCRs) were performed in an MX3000 detection system using SYBR green PCR reagents following the manufacturer's instructions (Stratagene). For each gene analyzed, a calibration curve was performed and all the oligonucleotides were tested to ensure specificity and sensitivity. Primers for C/EBPα and PU.1 were generated as described.12 These primers were used for the following sequences: SKP2-F (TGGGATCTTTTCCTGTCTGTG), SKP2-R (TACCCGGAAAGAGCTGAAGC); p21-F (GAGCCACAGGCACCATGTCC), p21-R (AGACCTTGGGCAGCCCTAGG); and LRG47-F (TGAGCTCAGCCTTCCCCTTT), LRG47-R (TGGGACAATGTTGCCACAGT).
Statistical analysis
Equality of distributions for matched pairs of observations was tested using the t test. Non-Gaussian distributed data were analyzed by the Wilcoxon signed-rank test using the StatPlus 2008 Professional software package (AnalystSoft). Error bars in panels represent SD, or SEM when the sample number is 10 or more.
Results
P aeruginosa infection decreases the number of neutrophils in the bone marrow
To study the changes occurring in the BM compartment during sepsis, we used an established murine model that recapitulates lethal sepsis in humans.7,8 In this model, a limited thermal injury in healthy animals followed by inoculation of the virulent strain of P aeruginosa UCBPP-PA14 (PA14; 2-3 × 105 CFU/mL) induces sepsis and approximately 90% mortality within 48 to 72 hours. Burn injury alone (B) is associated with modest local inflammation and 100% survival.7 We performed multiple trials of burn and inoculation with PA14 and collected BM, PB, and parenchymal tissue specimens at specific time points. Occurrence of sepsis during time was confirmed by the presence of PA14 in the blood (data not shown). PA14 infection induced a significant decrease of neutrophils in the circulation, as previously documented,13 coupled with the absence of inflammatory leukocyte infiltrates at the site of inoculation and in the parenchymal organs, which had extensive areas of necrosis due to bacteria presence (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article).
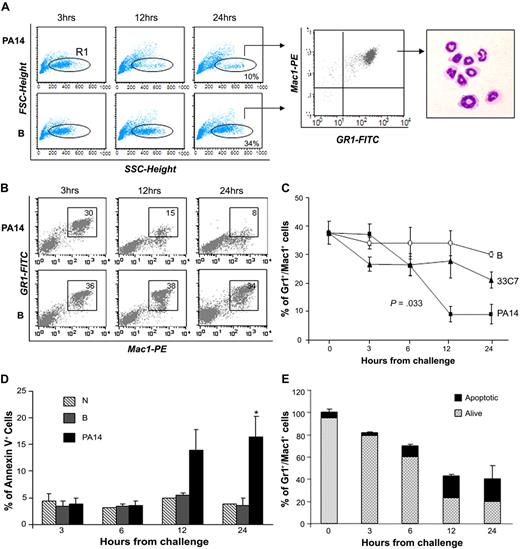
Total BM cellularity was progressively reduced in PA14-infected mice and decreased to 40% in comparison to normal controls by 24 hours (supplemental Figure 2). Immunophenotypic analysis indicated that the cellular loss was within the BM Gr1+Mac1+ population, which includes maturing myeloid cells, monocytes, and neutrophils. The decrease in mature neutrophils was confirmed by analysis of Gr1+Mac1+ cells with low forward scatter (FSC) and high side scatter (SSC), and by morphologic analysis of sorted populations (Figure 1A). The reduction of neutrophils in PA14-infected mice was progressive and greater than 70% at 24 hours, whereas in B mice, neutrophils levels in the BM decreased less than 10% (Figure 1B-C), likely due to trafficking to the site of thermal injury (supplemental Figure 1A). Next, we examined whether neutrophil loss was due to increased apoptosis during sepsis.14 Evaluation of apoptosis within the Gr1+Mac1+ population showed a higher percentage of annexin V–positive cells in PA14-infected mice at 12 and 24 hours (Figure 1D). However, the level of apoptosis (15%-20%) in PA14-infected mice suggests that apoptosis accounted partially for the neutrophils shortage observed during sepsis (Figure 1E). Evaluation of all mature subsets during sepsis confirmed that the cell loss was specific for Gr1+Mac1+ cells, whereas no substantial changes were observed in mature T, natural killer (NK) cells, and erythroid progenitors (Ter119+); B cells showed an increase (data not shown). These findings clearly show that P aeruginosa infection induces a profound depletion of neutrophils in the BM, resulting in a general state of neutropenia and lack of inflammatory infiltrates in the parenchymal organs.
Loss of bone marrow neutrophils during sepsis. CD1 mice challenged with burn and inoculation of the PA14 (PA14) or 33C7 (33C7) strain, burn only (B), and normal controls (N) were killed at the indicated time points. Bone marrow (BM) cells were harvested and stained with antibodies directed to Gr1 and Mac1 markers and analyzed by flow cytometry. (A) Dot blots in the left panel show FSC (indicative of size) and SSC (indicative of granularity) of total BM samples. The FCSlow/SSChigh population was gated (R1) and analyzed for Gr1/Mac1 expression (middle panel), and sorted for morphologic analysis (right panel). (B) Dot blots show Gr1 and Mac1 expression in a representative experiment. Numbers indicate percentage of cells within the gate. (C) Line graph summarizes 3 independent experiments. Values are the average of 6 to 10 mice and indicate percentages of BM Gr1+/Mac1+ cells. The value at time 0 is the average of 15 normal controls. Error bars indicate SD. (D) Bar graph shows percentages of annexin V expression on Gr1+/Mac1+ population in N, B, and PA14 mice. Values are averages of 3 to 4 mice. Error bars indicate SD. *P = .04. (E) Bar graph shows the average percentage of total BM Gr1+/Mac1+ cells in PA14-challenged mice; percentages are normalized to Gr1+/Mac1+ cells in normal controls to equal 100%. The light portion of the column represents the average percentage of living cells; the dark portion of the column represents average percentage of annexin V+ apoptotic cells (n = 4). Error bars indicate SD.
Loss of bone marrow neutrophils during sepsis. CD1 mice challenged with burn and inoculation of the PA14 (PA14) or 33C7 (33C7) strain, burn only (B), and normal controls (N) were killed at the indicated time points. Bone marrow (BM) cells were harvested and stained with antibodies directed to Gr1 and Mac1 markers and analyzed by flow cytometry. (A) Dot blots in the left panel show FSC (indicative of size) and SSC (indicative of granularity) of total BM samples. The FCSlow/SSChigh population was gated (R1) and analyzed for Gr1/Mac1 expression (middle panel), and sorted for morphologic analysis (right panel). (B) Dot blots show Gr1 and Mac1 expression in a representative experiment. Numbers indicate percentage of cells within the gate. (C) Line graph summarizes 3 independent experiments. Values are the average of 6 to 10 mice and indicate percentages of BM Gr1+/Mac1+ cells. The value at time 0 is the average of 15 normal controls. Error bars indicate SD. (D) Bar graph shows percentages of annexin V expression on Gr1+/Mac1+ population in N, B, and PA14 mice. Values are averages of 3 to 4 mice. Error bars indicate SD. *P = .04. (E) Bar graph shows the average percentage of total BM Gr1+/Mac1+ cells in PA14-challenged mice; percentages are normalized to Gr1+/Mac1+ cells in normal controls to equal 100%. The light portion of the column represents the average percentage of living cells; the dark portion of the column represents average percentage of annexin V+ apoptotic cells (n = 4). Error bars indicate SD.
P aeruginosa infection induces expansion of the LSK cell pool in the bone marrow
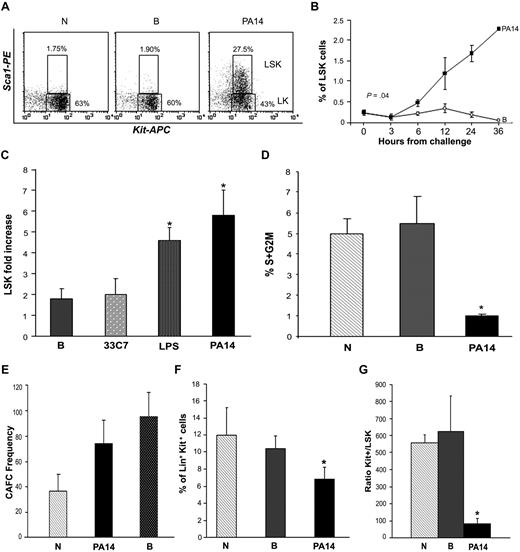
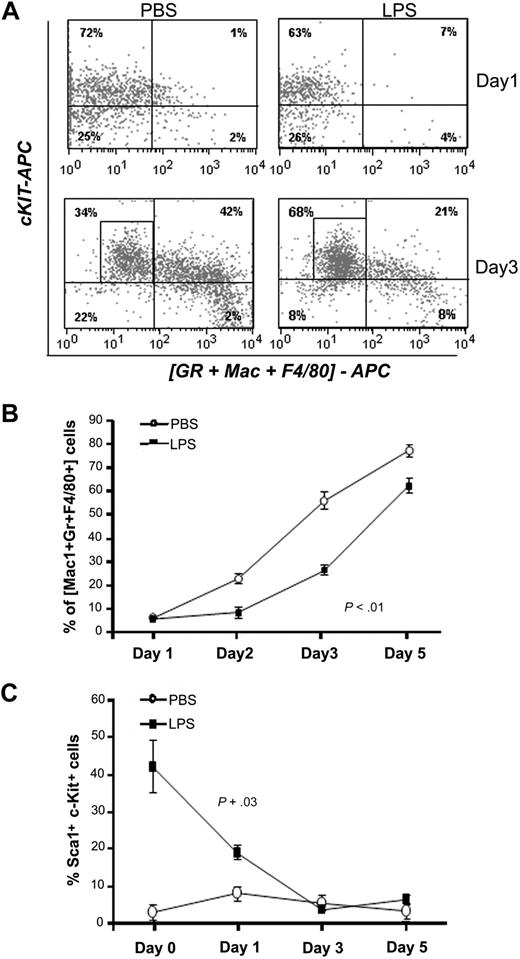
Neutrophils have a short half-life, and their destruction needs to be continuously compensated by rapid regeneration by the progenitor and stem cell compartment.3 To test whether the exhaustion of the BM neutrophil pool during sepsis was due to a defect in regeneration, we examined the BM progenitor/stem cell compartment by immunophenotypic analysis.15 This analysis demonstrated that LSK cells (cells negative for lineage markers [Lin−] and positive for Sca1 and c-Kit), a cell subset greatly enriched for hematopoietic stem and progenitor cells (HSPCs), were significantly increased in PA14-infected mice at 24 hours from bacterial challenge, both as percentage and in absolute number (5- to 8-fold increase; Figure 2A-C; supplemental Figure 2B). Interestingly, analysis of the cell cycle showed that LSK cells of septic animals were in a more quiescent state (Figure 2D). Enrichment of the more quiescent and primitive pool was also substantiated by increase in the frequency of CAFCs in the BM of septic mice (Figure 2E). Despite the significant expansion of the LSK pool, the pool of progenitor Lin+/Sca1−/Kit+ cells (Kit+; enriched in myeloid and erythroid progenitors) was reduced by 40% in PA14-infected mice (Figure 2F), and the ratio of progenitors to HSPCs (Kit+/LSK) was significantly lower in the BM of PA14-challenged mice compared with controls (Figure 2G), suggesting a block of differentiation.
Sepsis induces increase of primitive cells with LSK phenotype. CD1 mice challenged with burn and inoculation of the PA14 (PA14) or 33C7 (33C7) strain, burn only (B), and normal controls (N) were killed at the indicated time points. BM cells were stained with antibodies directed to Lin+ markers and to Sca1 and c-Kit markers. Samples were analyzed by multicolor flow cytometric analysis. (A) Dot blots show Sca1 and c-Kit expression on gated Lin− cells in N, B, and PA14 mice at 24 hours after challenge in a representative experiment. Numbers indicate percentage of cells within the Lin− gate. (B) Line graph indicates percentage of LSK cells on overall BM with exclusion of the R1 region in Figure 1A (to avoid relative increase of percentage due to loss of the FCSlow/SSChigh population) in a representative experiment. Values are averages of 4 to 8 mice. Time 0 is the average of 8 normal animals. (C) Bar graph shows average fold increase in LSK absolute number at 24 hours from challenge. In each column values are the average of 10 mice compared with normal controls in 3 independent experiments. Error bars indicate SE. LPS: *P = .03; PA14: *P = .01. (D) Bar graph indicates percentages of LSK cells in the S+G2M phase of the cell cycle, as determined by Hoechst staining. Values indicate average of 3 mice at 24 hours from challenge in a representative experiment. Error bars indicate SD. *P < .01. (E) Long-term culture with limiting dilution was performed on BM cells to quantify the frequencies of hematopoietic progenitors and stem cells. Data indicate frequency of CAFCs and are represented as means ± SD. (F) Bar graph indicates percentage of Lin+ c-Kit+ cells in N, B, and PA14 mice at 24 hours from challenge. Values indicate average of 6 mice in a representative experiment at 24 hours from challenge. Error bars indicate SD. *P < .001. (G) Measure of LSK cell output. Bar graph shows ratio of percentages of Lin+Kit+ (Kit+) over LSK cells (r = Kit+/LSK). Percentages of each population were measured on identical gates to equal total BM excluding R1, as in Figure 1A. Values indicate average of 6 mice in 2 independent experiments at 24 hours from challenge. Error bars indicate SD. *P = .016.
Sepsis induces increase of primitive cells with LSK phenotype. CD1 mice challenged with burn and inoculation of the PA14 (PA14) or 33C7 (33C7) strain, burn only (B), and normal controls (N) were killed at the indicated time points. BM cells were stained with antibodies directed to Lin+ markers and to Sca1 and c-Kit markers. Samples were analyzed by multicolor flow cytometric analysis. (A) Dot blots show Sca1 and c-Kit expression on gated Lin− cells in N, B, and PA14 mice at 24 hours after challenge in a representative experiment. Numbers indicate percentage of cells within the Lin− gate. (B) Line graph indicates percentage of LSK cells on overall BM with exclusion of the R1 region in Figure 1A (to avoid relative increase of percentage due to loss of the FCSlow/SSChigh population) in a representative experiment. Values are averages of 4 to 8 mice. Time 0 is the average of 8 normal animals. (C) Bar graph shows average fold increase in LSK absolute number at 24 hours from challenge. In each column values are the average of 10 mice compared with normal controls in 3 independent experiments. Error bars indicate SE. LPS: *P = .03; PA14: *P = .01. (D) Bar graph indicates percentages of LSK cells in the S+G2M phase of the cell cycle, as determined by Hoechst staining. Values indicate average of 3 mice at 24 hours from challenge in a representative experiment. Error bars indicate SD. *P < .01. (E) Long-term culture with limiting dilution was performed on BM cells to quantify the frequencies of hematopoietic progenitors and stem cells. Data indicate frequency of CAFCs and are represented as means ± SD. (F) Bar graph indicates percentage of Lin+ c-Kit+ cells in N, B, and PA14 mice at 24 hours from challenge. Values indicate average of 6 mice in a representative experiment at 24 hours from challenge. Error bars indicate SD. *P < .001. (G) Measure of LSK cell output. Bar graph shows ratio of percentages of Lin+Kit+ (Kit+) over LSK cells (r = Kit+/LSK). Percentages of each population were measured on identical gates to equal total BM excluding R1, as in Figure 1A. Values indicate average of 6 mice in 2 independent experiments at 24 hours from challenge. Error bars indicate SD. *P = .016.
To determine the physiologic relevance of these observations, parallel trials were conducted with an isogenic nonlethal PA14 mutant, 33C7.16 In these trials, survival was associated with presence of leukocyte infiltrates in the peripheral organs and lack of the dramatic HSPC expansion observed during infection with the PA14 strain. Analysis of the BM of mice infected with the 33C7 nonlethal mutant strain showed only a modest increase of LSK cells (2-fold at 24 hours), similar to that observed in mice subjected to burn injury only (Figure 2C; supplemental Figure 2B). This correlated with a modest decrease of the number of neutrophil levels in the BM (Figure 1C), likely due to migration and abundant infiltration of leukocytes in the peripheral organs until resolution of the infection (supplemental Figure 1B).
Overall, these findings demonstrate that P aeruginosa infection induces an abnormal expansion of LSK cells coupled with a profound depletion of BM neutrophils and suggest that their regeneration is impaired by a block of differentiation.
P aeruginosa LPS is sufficient to cause BM neutrophil depletion and LSK pool expansion
Next, we investigated the mechanisms triggering the BM effects during infection. Despite the large number of studies on the role of bacterial LPS in the host immune response,17 its in vivo effects on HSPCs and progenitors have not been investigated. To test whether LPS from P aeruginosa was critical and sufficient to induce the BM phenotype observed during sepsis, we injected increasing concentrations of purified P aeruginosa LPS into mice. Analysis of BM showed that injection of 0.8 mg/kg (comparable to the amount of P aeruginosa's LPS present in the blood at 24 to 30 hours from inoculation with live bacteria) was sufficient to induce the same effects observed during bacterial sepsis. Similar to the observations during PA14 infection, P aeruginosa's LPS induced a decrease in neutrophils and an increase in LSK cells (Figures 2C, 3A, and 4A). Histologic analysis of the BM confirmed hypocellularity with visible decrease in neutrophils and increased frequency of immature forms (Figure 3B). As observed during sepsis in PA14-infected mice, LSK cells from LPS-challenged mice showed reduced cell cycle (Figure 3C), further confirmed by evidence of transcriptional down-regulation of SKP2, a key regulator promoting cell-cycle entry18 (Figure 3D). Similar results were found on Lin− cells (supplemental Figure 3A-D). In agreement with the increased number of immature cells, BM from LPS-challenged mice showed a higher frequency of primitive cells capable of generating CAFCs (Figure 2E), and a lower ability to generate colonies derived from more committed progenitors (CFU-C; Figure 3E).
P aeruginosa's LPS is sufficient and necessary to induce the BM alterations observed during sepsis. Cohorts of CD1 and C57BL/6J mice were injected intraperitoneally with P aeruginosa's LPS (0.8 mg/kg) or phosphate-buffered saline (PBS) and compared with mice challenged with PA14. Mice were killed at the indicated time points, and their BM cells were analyzed. (A) BM cells harvested at 24 hours from LPS or PBS injection, or PA14 challenge, were labeled with antibodies anti-Gr1 and anti-Mac1. Bar graph shows percentage of Gr1+/Mac1+ cells in total BM. Values are average of 6 mice in 3 independent experiments. Error bars indicate SD. *P < .001. (B) BM smears from PBS- or LPS-challenged mice. Samples were stained by hematoxylin/eosin, and morphology was evaluated by light microscopy (×100 magnification). Circled area indicates the presence of neutrophils in PBS condition and of immature cells in LPS (arrows). (C) Bar graph indicates percentages of LSK cells in the S+G2M phase of the cell cycle, as determined by Hoechst staining. Values indicate average of 2 mice at 24 hours from challenge in a representative experiment. Error bars indicate SD. (D) Each sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice at 24 hours. Samples were analyzed for expression of SKP2 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples from 2 independent experiments. Error bars indicate SD: LPS versus PBS, P = .04. (E) BM cells were analyzed for colony-forming ability by methylcellulose colony assay. Bar graph shows average number of myeloid colonies (CFU-GM + CFU-G + CFU-M) per 10 000 cells in 3 independent samples, each of them in quadruplicate. *P < .001.
P aeruginosa's LPS is sufficient and necessary to induce the BM alterations observed during sepsis. Cohorts of CD1 and C57BL/6J mice were injected intraperitoneally with P aeruginosa's LPS (0.8 mg/kg) or phosphate-buffered saline (PBS) and compared with mice challenged with PA14. Mice were killed at the indicated time points, and their BM cells were analyzed. (A) BM cells harvested at 24 hours from LPS or PBS injection, or PA14 challenge, were labeled with antibodies anti-Gr1 and anti-Mac1. Bar graph shows percentage of Gr1+/Mac1+ cells in total BM. Values are average of 6 mice in 3 independent experiments. Error bars indicate SD. *P < .001. (B) BM smears from PBS- or LPS-challenged mice. Samples were stained by hematoxylin/eosin, and morphology was evaluated by light microscopy (×100 magnification). Circled area indicates the presence of neutrophils in PBS condition and of immature cells in LPS (arrows). (C) Bar graph indicates percentages of LSK cells in the S+G2M phase of the cell cycle, as determined by Hoechst staining. Values indicate average of 2 mice at 24 hours from challenge in a representative experiment. Error bars indicate SD. (D) Each sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice at 24 hours. Samples were analyzed for expression of SKP2 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples from 2 independent experiments. Error bars indicate SD: LPS versus PBS, P = .04. (E) BM cells were analyzed for colony-forming ability by methylcellulose colony assay. Bar graph shows average number of myeloid colonies (CFU-GM + CFU-G + CFU-M) per 10 000 cells in 3 independent samples, each of them in quadruplicate. *P < .001.
In conclusion, our data show that LPS from P aeruginosa is sufficient to induce the effects observed in the BM during P aeruginosa sepsis. Given the causal role of LPS in determining the BM phenotype and the challenges of manipulating bacterial-infected BM cells, further analysis of the BM compartment was performed using P aeruginosa's LPS inoculation as surrogate for the sepsis model.
P aeruginosa's LPS induces reduction of BM multipotential progenitors, CMPs and GMPs, and defective myeloid differentiation
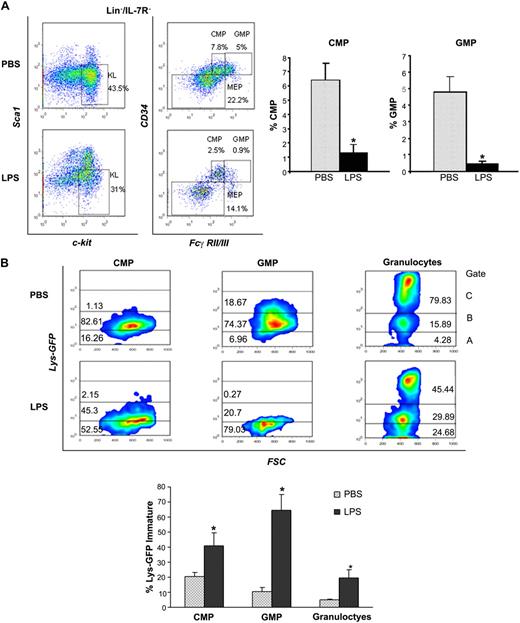
To address the mechanisms involved in the differentiation block, we characterized the stem and progenitor subsets and measured the egress from the LSK pool into the more mature pool of CMPs and GMPs, which give rise to the myeloid progeny and ultimately to neutrophils. Analysis of distinct Lin− subsets by multiparameter immunophenotypic analysis19 demonstrated that LPS caused a dramatic reduction of CMPs (Lin−IL-7R−Sca−Kit+CD34+FcγRII/IIIlow) and GMPs (Lin−IL-7R−Sca−Kit+CD34+FcγRII/IIIhi; Figure 4A-B). Apoptosis evaluation revealed negligible differences in these subsets in the 2 conditions (data not shown). Next, we compared the rates of progenitor differentiation by analysis of lysozyme expression, a marker of myeloid differentiation increasingly expressed from CMPs to neutrophils.20 To this end, we used Lys-EGFP reporter mice in which GFP expression is driven by the lysozyme promoter.9 Lys-EGFP mice challenged with P aeruginosa's LPS showed that the CMP, GMP, and neutrophils had a much lower expression of lysozyme than controls, with prevalence of immature forms (Lys-EGFP–negative or dim) in each subset (Figure 4B), confirming lower rates of myeloid differentiation. Evaluation of the Lys-EGFP expression in LSK cells confirmed a similar expression in PBS- and LPS-challenged mice, thus excluding the possibility that the expansion of LSK pool is due to the CMP's and GMP's failure to down-regulate Sca1 (supplemental Figure 4A).
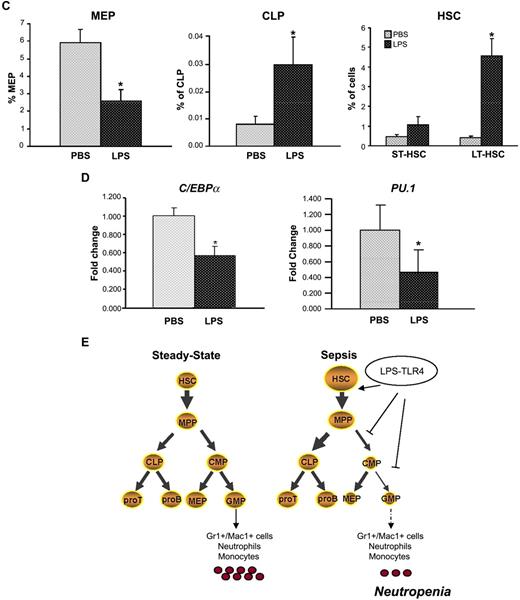
P aeruginosa's LPS causes reduction of BM common myeloid progenitors and granulocyte-monocyte progenitors. Cohorts of CD1 or C57BL/6J mice were injected intraperitoneally with P aeruginosa's LPS (0.8 mg/kg) or PBS. (A) BM was harvested 24 hours after injection, stained with specific antibodies, and analyzed by multicolor flow cytometry. The dot blot on the left insight shows expression of c-Kit and Sca-1 in Lin−/IL-7R− cells. Dot blot on the right insight shows FcγRII/III expression on gated Lin−/IL7-R−/Sca−/Kit+ cells to determine CMP, GMP, and MEP subsets. Bar graphs indicate percentages of CMPs and GMPs on the Lin− population. Values are average of 10 to 12 mice and summarize 3 independent experiments. Error bars indicate SE. CMP: LPS versus PBS *P < .001; GMP: LPS versus PBS *P < .001. (B) Lys-GFP reporter mice were challenged with P aeruginosa's LPS or PBS, and the BM was harvested at 24 hours from injection. Dot plots show GFP expression on CMPs, GMPs, and granulocytes in a representative experiment. Values in the bar graph indicate percentage of cells that express no or low levels of Lys-GFP (gate A) within each specific subset and are average of 4 mice. CMP: LPS versus PBS *P = .05; GMP: LPS versus PBS *P < .001; granulocytes: LPS versus PBS *P = .035. (C) BM of C57/B6 mice challenged with LPS or PBS was analyzed for the presence of MEPs (left panel), CLPs (middle panel), and MPPs (right panel) at 24 hours from challenge. Bar graphs indicate percentages of MEPs and CLPs on the Lin− population. Values are average of 10 to 12 mice and summarize 3 independent experiments. Error bars indicate SE. MEP: LPS versus PBS *P < .001; CLP: LPS versus PBS *P < .001. Short-term hematopoietic stem cells (ST-HSCs; or MPPs) were defined as Lin−/IL-7R−/Sca+/Kit+/CD34+/Flt3+ cells. Bar graph shows percentage of ST-HSCs ( ) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
P aeruginosa's LPS causes reduction of BM common myeloid progenitors and granulocyte-monocyte progenitors. Cohorts of CD1 or C57BL/6J mice were injected intraperitoneally with P aeruginosa's LPS (0.8 mg/kg) or PBS. (A) BM was harvested 24 hours after injection, stained with specific antibodies, and analyzed by multicolor flow cytometry. The dot blot on the left insight shows expression of c-Kit and Sca-1 in Lin−/IL-7R− cells. Dot blot on the right insight shows FcγRII/III expression on gated Lin−/IL7-R−/Sca−/Kit+ cells to determine CMP, GMP, and MEP subsets. Bar graphs indicate percentages of CMPs and GMPs on the Lin− population. Values are average of 10 to 12 mice and summarize 3 independent experiments. Error bars indicate SE. CMP: LPS versus PBS *P < .001; GMP: LPS versus PBS *P < .001. (B) Lys-GFP reporter mice were challenged with P aeruginosa's LPS or PBS, and the BM was harvested at 24 hours from injection. Dot plots show GFP expression on CMPs, GMPs, and granulocytes in a representative experiment. Values in the bar graph indicate percentage of cells that express no or low levels of Lys-GFP (gate A) within each specific subset and are average of 4 mice. CMP: LPS versus PBS *P = .05; GMP: LPS versus PBS *P < .001; granulocytes: LPS versus PBS *P = .035. (C) BM of C57/B6 mice challenged with LPS or PBS was analyzed for the presence of MEPs (left panel), CLPs (middle panel), and MPPs (right panel) at 24 hours from challenge. Bar graphs indicate percentages of MEPs and CLPs on the Lin− population. Values are average of 10 to 12 mice and summarize 3 independent experiments. Error bars indicate SE. MEP: LPS versus PBS *P < .001; CLP: LPS versus PBS *P < .001. Short-term hematopoietic stem cells (ST-HSCs; or MPPs) were defined as Lin−/IL-7R−/Sca+/Kit+/CD34+/Flt3+ cells. Bar graph shows percentage of ST-HSCs ( ) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
Global analysis of Lin− subpopulations also revealed a decrease in megakaryocytic-erythroid progenitors (MEPs; Lin−IL-7R−Sca−Kit+CD34−FcγRII/IIIlo; Figure 4A,C), which, however, was not associated with decreased erythroid progenitors, and an increase in common lymphoid progenitors (CLPs; Figure 4C). Further distinction of the LSK subsets in LSK IL-7R−CD34+FLT3+ (multipotential progenitors [MPPs]; short-term HSCs [ST-HSCs]) and IL-7R−CD34−FLT3− (long-term HSCs [LT-HSCs]), indicated that LPS induced a greater increase in cells with LT-HSC phenotype (average, 4.75% vs 0.43% control) than in cells with ST-HSC phenotype (average, 1.0% vs 0.47% control). Quantitative reverse transcriptase–PCR (qRT-PCR) analysis of PU.1 and C/EBPα, transcription factors involved in myeloid differentiation, showed that they were expressed at significant lower levels in LSK cells sorted from LPS-challenged mice than in controls (Figure 4D).
To confirm that the altered representation of LSK, MPPs, CMPs, and GMPs had an impact on differentiation dynamics, Lin− cells purified from LPS mice or PBS controls were tested for their ability to differentiate under myelodifferentiative conditions in vitro. Lin− cells from controls rapidly generated differentiated myeloid cells expressing Mac1, Gr1, and F4/80 (Figure 5A-B). In contrast, Lin− cells from LPS-challenged mice maintained high levels of the primitive markers c-Kit and Sca1 during that time and required more than 5 days to generate an equivalent proportion of mature myeloid cells (Figure 5C). In conclusion, these data suggest that during sepsis, P aeruginosa LPS skews primitive hematopoietic cells toward a more immature state, characterized by increased numbers of LSK cells and decreased output into the CMP and GMP pools, resulting in a lower ability to progress toward myeloid differentiation. Because neutrophils have a very short life span, low efficiency in myeloid differentiation has a greater impact on their levels, as clearly visible in vivo during sepsis.
P aeruginosa's LPS causes defective myeloid differentiation. C57BL/6J mice were injected intraperitoneally with P aeruginosa's LPS (0.8 mg/kg) or PBS. BM was harvested at 24 hours from injection, and Lin− cells were sorted by FACS and grown in vitro in prodifferentiative conditions in the presence of SCF, IL-3, and G-CSF. Cells were harvested at the indicated time points and analyzed for expression of differentiation markers by flow cytometry. (A) Dot blots shows expression of c-Kit and of a combination of myeloid differentiation markers (Gr1 + Mac1 + F4/80) at day 1 and day 3 of culture in a representative experiment. (B) Line graph represents acquisition of the myeloid markers (Gr1, Mac1, F4/80) over time. Values represent average percentages of 3 samples from a representative experiment of 3 independent experiments. Error bars indicate SD. Day 3 *P < .001. (C) Line graph shows kinetic of LSK cells in culture in 3 independent experiments. Values represent average percentage of LSK cells (n = 7).
P aeruginosa's LPS causes defective myeloid differentiation. C57BL/6J mice were injected intraperitoneally with P aeruginosa's LPS (0.8 mg/kg) or PBS. BM was harvested at 24 hours from injection, and Lin− cells were sorted by FACS and grown in vitro in prodifferentiative conditions in the presence of SCF, IL-3, and G-CSF. Cells were harvested at the indicated time points and analyzed for expression of differentiation markers by flow cytometry. (A) Dot blots shows expression of c-Kit and of a combination of myeloid differentiation markers (Gr1 + Mac1 + F4/80) at day 1 and day 3 of culture in a representative experiment. (B) Line graph represents acquisition of the myeloid markers (Gr1, Mac1, F4/80) over time. Values represent average percentages of 3 samples from a representative experiment of 3 independent experiments. Error bars indicate SD. Day 3 *P < .001. (C) Line graph shows kinetic of LSK cells in culture in 3 independent experiments. Values represent average percentage of LSK cells (n = 7).
Septic LSK cells show decreased short-term engraftment and reduced long-term repopulating ability
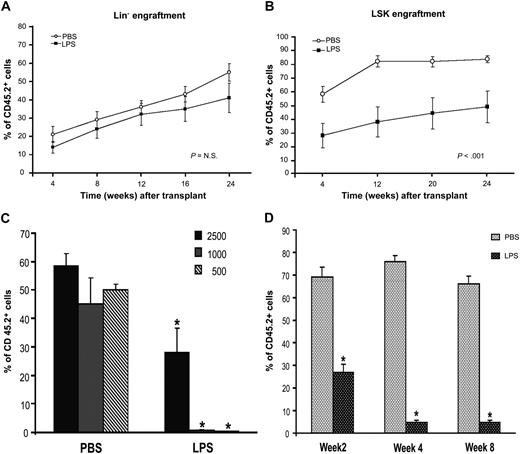
We examined the functional properties of the “septic” HSPCs by competitive BM transplantation assays. Equivalent numbers of CD45.2 Lin− cells sorted from BM of PBS- or LPS-challenged mice were transplanted into lethally irradiated CD45.1 recipients together with competitor cells (CD45.1). Engraftment analysis by PB chimerism (CD45.2 vs CD45.1) was performed at successive 4-week time points from transplantation. As the Lin− cells derived from LPS-challenged mice contained higher percentages of LSK cells than controls, we expected to observe a significant increase in the long-term engraftment ability of this pool in transplanted animals. However, Lin− cells from LPS-challenged mice showed a long-term repopulating ability not significantly different from the Lin− cells of controls (Figure 6A), indicating no improvement in overall stem cell activity. Multilineage analysis performed in the PB in conjunction with CD45.1 and CD45.2 markers at 6, 9, and 12 months showed that all blood lineages were reconstituted in both groups (data not shown).
Septic HSPCs show functional defects in BM transplantation assays. C57BL/6J CD45.2 mice were injected intraperitoneally with LPS from P aeruginosa (0.8 mg/kg) or PBS. BM was harvested at 24 hours from injection, and Lin− or LSK cells, IL-7R− (to exclude contamination from CLPs), were sorted by FACS. Sorted populations were transplanted into irradiated CD45.1 donors, and engraftment was evaluated at each indicated time point by analysis of CD45.2+ cells in the peripheral blood. (A) A total of 2 × 105 Lin−CD45.2+ sorted cells were injected into a lethally irradiated CD45.1+ Boy/J recipient together with 3 × 105 Lin−CD45.1+ competitor cells. Line graph shows engraftment as percentage of CD45.2 cells during time. Values are average of 10 to 12 mice from 3 independent experiments. Bars indicate SE. P value is nonsignificant. (B) A total of 2500 LSK/CD45.2+ sorted cells were injected into a lethally irradiated CD45.1 Boy/J recipient together with 105 CD45.1+ competitor total BM cells. Line graph shows engraftment as percentage of CD45.2 cells during time. Values are average of 10 to 12 mice from 3 independent experiments. Bars indicate SE. *P < .001. (C) Bar graph shows level of donor engraftment in the PB at 4 weeks of transplantation in mice receiving decreasing doses (2500, 1000, 500) of LSK cells derived from PBS controls or LPS-challenged mice. Values are average of 5 to 8 mice. *P < .001. (D) PB cells were collected and stained with antibodies directed to the myeloid lineage. Bar graph shows percentages of donors Gr1+/CD45.2+ cells on total GR1+ cells (100%) at 2, 4, and 8 weeks from transplantation. Values are average of 8 to 12 mice. Error bars indicate SE. *P < .001.
Septic HSPCs show functional defects in BM transplantation assays. C57BL/6J CD45.2 mice were injected intraperitoneally with LPS from P aeruginosa (0.8 mg/kg) or PBS. BM was harvested at 24 hours from injection, and Lin− or LSK cells, IL-7R− (to exclude contamination from CLPs), were sorted by FACS. Sorted populations were transplanted into irradiated CD45.1 donors, and engraftment was evaluated at each indicated time point by analysis of CD45.2+ cells in the peripheral blood. (A) A total of 2 × 105 Lin−CD45.2+ sorted cells were injected into a lethally irradiated CD45.1+ Boy/J recipient together with 3 × 105 Lin−CD45.1+ competitor cells. Line graph shows engraftment as percentage of CD45.2 cells during time. Values are average of 10 to 12 mice from 3 independent experiments. Bars indicate SE. P value is nonsignificant. (B) A total of 2500 LSK/CD45.2+ sorted cells were injected into a lethally irradiated CD45.1 Boy/J recipient together with 105 CD45.1+ competitor total BM cells. Line graph shows engraftment as percentage of CD45.2 cells during time. Values are average of 10 to 12 mice from 3 independent experiments. Bars indicate SE. *P < .001. (C) Bar graph shows level of donor engraftment in the PB at 4 weeks of transplantation in mice receiving decreasing doses (2500, 1000, 500) of LSK cells derived from PBS controls or LPS-challenged mice. Values are average of 5 to 8 mice. *P < .001. (D) PB cells were collected and stained with antibodies directed to the myeloid lineage. Bar graph shows percentages of donors Gr1+/CD45.2+ cells on total GR1+ cells (100%) at 2, 4, and 8 weeks from transplantation. Values are average of 8 to 12 mice. Error bars indicate SE. *P < .001.
Next, we evaluated the specific competitive repopulating activity and differentiation ability of LSK cells. LSK cells sorted from BM of LPS-challenged mice showed a significant lower level of engraftment in the long term (3 to 12 months) and in the short term (4 to 12 weeks); this difference was further increased when lower cell doses were used for transplantation (Figure 6B-C). Multilineage analysis of the peripheral blood of animals that underwent transplantation showed a dramatic decrease of donor Gr1+ cells compared with competitor (Figure 6D), accompanied by absence of CMPs and GMPs in the BM (data not shown), confirming a defective ability of the “septic” LSK to generate CMPs, GMPs, and mature myeloid cells. Taken together, these results show that during sepsis, the dramatic immunophenotypic expansion of both ST-HSCs and LT-HSCs is associated with defective stem cell activity and decreased ability to generate CMPs and GMPs.
Loss of TLR4 rescue the myeloid differentiation block observed during sepsis
TLR4 is a critical component in cellular recognition and activation by LPS.17 TLR4 is expressed by several cell types in the BM, including LSK cells.21 Interestingly, we observed that TLR4 expression was significantly up-regulated in LSK cells during LPS challenge in vivo (Figure 7A). Furthermore, LSK cells from LPS-treated mice showed transcriptional down-regulation of IKBα, indicating their ability to participate to the TLR4/NF-κB signaling circuitry (supplemental Figure 4B).
The effects induced by LPS on HSCs and neutrophils are abrogated in a TLR4-null phenotype. (A) C57BL/6J CD45.2 mice were injected intraperitoneally with LPS from P aeruginosa (0.8 mg/kg) or PBS. BM was harvested at 24 hours from injection, and Lin− cells were purified and stained with c-Kit, Sca1, and TLR4/MD2 antibodies. Histograms show TLR4 expression on LSK cells (black line) superimposed to TLR4 expression on LK cells (Lin−Kit+ Sca−; gray filled curve) in PBS control (left panel) and in LPS-challenged mice (right panel). TLR4 expression on LK cells was at limit of detection and superimposed with IgGs control. (B) C3H/OuJ(TLR4wt) and C3H/HeJ (TLR4−) mice were injected intraperitoneally with LPS from P aeruginosa (0.8 mg/kg) or PBS. BM was harvested at 24 hours, stained with monoclonal antibodies necessary to identify the indicated subsets, and analyzed by multicolor flow cytometric analysis. (B) Average percentage of LSK on the Lin− cells. TLR4WT: PBS versus LPS; *P = .045. (C) Average percentage of Gr1+/Mac1+ neutrophils in the total BM population. TLR4WT: PBS versus LPS *P < .001. (D) Average percentage of CMPs in the Lin− population. TLR4WT: PBS versus LPS; *P < .001. (E) Average percentage of GMPs in the Lin− population. TLR4WT: PBS versus LPS; *P < .001. In all graphs, values are average of 6 mice from 2 independent experiments. Error bars show SD.
The effects induced by LPS on HSCs and neutrophils are abrogated in a TLR4-null phenotype. (A) C57BL/6J CD45.2 mice were injected intraperitoneally with LPS from P aeruginosa (0.8 mg/kg) or PBS. BM was harvested at 24 hours from injection, and Lin− cells were purified and stained with c-Kit, Sca1, and TLR4/MD2 antibodies. Histograms show TLR4 expression on LSK cells (black line) superimposed to TLR4 expression on LK cells (Lin−Kit+ Sca−; gray filled curve) in PBS control (left panel) and in LPS-challenged mice (right panel). TLR4 expression on LK cells was at limit of detection and superimposed with IgGs control. (B) C3H/OuJ(TLR4wt) and C3H/HeJ (TLR4−) mice were injected intraperitoneally with LPS from P aeruginosa (0.8 mg/kg) or PBS. BM was harvested at 24 hours, stained with monoclonal antibodies necessary to identify the indicated subsets, and analyzed by multicolor flow cytometric analysis. (B) Average percentage of LSK on the Lin− cells. TLR4WT: PBS versus LPS; *P = .045. (C) Average percentage of Gr1+/Mac1+ neutrophils in the total BM population. TLR4WT: PBS versus LPS *P < .001. (D) Average percentage of CMPs in the Lin− population. TLR4WT: PBS versus LPS; *P < .001. (E) Average percentage of GMPs in the Lin− population. TLR4WT: PBS versus LPS; *P < .001. In all graphs, values are average of 6 mice from 2 independent experiments. Error bars show SD.
To determine whether TLR4 signaling was required for the BM alterations observed in our model, we injected LPS from P aeruginosa in C3H/HeJ mice, which have a spontaneous mutation in the TLR4 gene resulting in its loss of function (TLR4−). The results were compared with C3H/OuJ mice, which have functional TLR4 receptors (TLR4wt). As anticipated, TLR4wt mice responded to LPS challenge with a significant decrease in neutrophil levels and a great expansion of LSK cells. In contrast, in TLR4− mice, LSK cells were not significantly increased, and neutrophils levels remained unchanged (Figure 7B-C). The modest expansion of LSK cells and the partial normalization of CMP and GMP in TLR4− mice challenged with LPS is likely due to TLR2 activation by residual peptidoglycan-associated lipoprotein (PAL) contaminating the LPS (data not shown).21,22 Further analysis of the CMP and GMP subsets showed that these progenitor pools were greatly protected by loss of TLR4 signaling in TLR4− mice challenged by LPS (Figure 7D-E). In conclusion, TLR4 loss greatly reduces the myeloid suppression induced by LPS.
Discussion
The results described here provide the first evidence of the physiologic mechanism causing neutropenia during lethal sepsis. Using an in vivo model of P aeruginosa infection, we show that the neutropenia occurring during sepsis is caused in part by apoptosis and is sustained by a block of HSPC differentiation. Specifically, we demonstrated that in response to the high consumption of mature cell production during sepsis, the HSPCs expand at the expense of myeloid differentiation, resulting in the dramatic reduction of CMPs and GMPs, the common myeloid progenitors that produce neutrophils (Figure 4E). Moreover, we found that these effects are TLR4 dependent. More broadly, our data establish a link between HSC regulation and host response in severe sepsis, a previously unrecognized connection.
One of the major challenges in this area of study is represented by the variability and limitations of the existing experimental models of sepsis in recapitulating the human pathophysiologic conditions.23 Thus, it is very important that the results are viewed in the context of the in vivo model of infection used.7,8,24 There is evidence that the mechanisms regulating neutrophil production and migration may change considerably with the severity of sepsis within the same experimental model.25,26 The burn P aeruginosa inoculation model used in this study closely resembles the late stages of sepsis in patients with overwhelming Gram-negative bacteremia. This model has a very brief early phase of hyperresponsive inflammatory response and is characterized by rapid onset of neutropenia, immunoparalysis, and death.8,13,24 In contrast, models of sublethal multimicrobial sepsis, such as cecal ligation and puncture or chronic infections, are characterized by a longer hyperactive immunoresponse and by a significant myelostimulation with increase of myeloid progenitors and neutrophils.27,28
Previous studies conducted on the burn P aeruginosa model revealed the existence of myelosuppression in addition to neutrophil consumption and apoptosis during sepsis.13,29 Interestingly, a recent study from Zhu et al showed that Listeria monocytogences, a Gram-positive bacteria with a TLR4 agonist component,30,31 can induce myelosuppression and neutropenia correlating with lethality.32 Although these studies clearly indicated the presence of a myelosuppressive activity during lethal sepsis, they were limited to the analysis of progenitor colonies and terminal differentiation of BM myeloid cells,33,34 and the mechanisms mediating this process were not elucidated. To address this question, we investigated how the stem and progenitor dynamics were altered during P aeruginosa–induced sepsis. A central finding of our work is the discovery that the common myeloid progenitors, CMP and GMP, which give rise to granulocytes and monocytes, are dramatically decreased during sepsis. Their decrease does not seem to be caused by cell death, as apoptosis evaluation in these subsets did not detect substantial differences between control and septic animals. Furthermore, CMPs and GMPs of septic animals are not only altered in numbers but also in their progress toward differentiation, as indicated by analysis of the lysozyme's expression.20 Using a transgenic Lys-EGFP reporter mouse model,9 we show that “septic” myeloid progenitors are characterized by a much lower expression of Lys-EGFP at all stages, from CMP to neutrophils, indicating delayed kinetic of differentiation. Overall, our data indicate that the decline in BM neutrophils observed in our model can largely be attributed to the decrease of common progenitors, although we do not exclude the possibility that other factors may contribute to the inhibition of myeloid differentiation at later stages.
Interestingly, we found that during sepsis the MEP subset, which contains erythroid multipotential progenitors, was significantly decreased without resulting in decreased Ter119 erythroid progenitors, supporting the notion that these cells can be also generated directly by the LSK fraction.35 In contrast, CLPs were significantly increased and correlated with augmented levels of B220 cells in the BM (data not shown).
What causes the reduction of common myeloid progenitors during sepsis? Alteration of the common progenitor subsets during sepsis was associated with abnormal expansion of the LSK pool. LSK increased 5- to 8-fold in absolute numbers per femur within 24 hours. Surprisingly, this expansion was not associated with increased cell cycle, but with a block of differentiation. LSK cells showed a more quiescent status with evidence of decreased expression of SKP2, a positive cell-cycle regulator.18 This observation was extended also to the progenitor pool of Lin− cells, characterized by decreased BrDU incorporation, decreased SKP2, and an increase in the cell-cycle inhibitor p21cip1. Although this finding was unexpected, it is supported by the recent observation that expansion of HSCs after 5FU is associated with up-regulation of LRG47, a negative regulator of cell proliferation.28 Interestingly, the GTPase LRG-47 is involved in host defense and has been shown to mediate hematopoietic recovery after bacterial infection.28 In our study, we have also confirmed the increased expression of LRG47 in Lin− cells from LPS-challenged mice. Indeed, cell-cycle arrest is a protective mechanism and a common cellular response to stress and injury.36,37
In steady-state hematopoiesis, a delicate balance exists between the stem cell pool and the compartments of progressively more committed and differentiated cells of all lineages. Egress from a compartment to a more mature compartment is regulated by the combinatorial effects of microenvironmental cues, transcription factors, and cell-cycle regulation. Failure to orderly progress from one stage to another characterizes hematopoietic disorders such as myelodysplastic syndromes and leukemias, in which mature cell production (ie, neutrophils and monocytes) is impaired by both increased apoptosis and block of differentiation at the stem cell/progenitor level.38 Our results show a decrease of mature myeloid cells and myeloid common progenitors associated with LSK expansion, strongly suggesting a block of differentiation. Our hypothesis is further supported by the in vitro and in vivo functional characterization of “septic” LSK cells. Consistent with the finding of decreased CMPs and GMPs in septic animals, LSK from septic mice had significantly lower expression of C/EBPα and PU.1, transcription factors critical for myeloid differentiation,35 and were defective in generating CMPs and GMPs in vivo, resulting in very low levels of donor myeloid cells in the short term in competitive transplantation assays. Furthermore, stem cells from septic mice acquired myeloid differentiation markers significantly later than controls when cultured in vitro in prodifferentiative LPS-free conditions. Despite the increase of immunophenotypically defined LT-HSCs (Lin−IL-7R−Sca+Kit+CD34−FLT3−),35 functional analysis by competitive repopulation assay demonstrated that “septic” LSK cells have a significant defect in sustaining long-term engraftment. Engraftment of Lin− cells from LPS- or PBS-treated mice showed similar long-term reconstitution despite the greater than 10-fold increase in LSK content in the LPS-treated population. This is more clearly demonstrated in the subsequent experiments using equivalent numbers of LSK from LPS- and PBS-treated mice, in which LSK from septic mice displayed markedly lower repopulating ability. In conclusion, the majority of the LSK cells that expanded during sepsis are dysfunctional, as they do not display long-term repopulating ability and do not generate an adequate output of multipotential myeloid progenitors.
The cellular and molecular mechanisms inducing myeloid suppression may be potential targets for therapy aimed at preserving key cellular components of the host innate immune system during sepsis. This concept is supported by the study of BitMansour and colleagues, in which transplantation of HSCs in combination with myeloid progenitors showed protection against P aeruginosa sepsis.39 The effects of the isogenic P aeruginosa mutant provided us with critical insights. The nonlethal strains had in common LPS defects and resulted in infections characterized by the absence of LSK alterations and preservation of myeloid progenitors and neutrophils regeneration (N.C. and L.G.R., unpublished observations, May 2003). Despite the large number of studies on the role of bacterial LPS in the host immune response,17 its in vivo effects on HSPCs and progenitors have not been investigated. In this report, we show that injection of LPS from P aeruginosa is sufficient to induce in vivo the expansion of HSPCs and the decrease of myeloid progenitors and neutrophils observed during P aeruginosa infection. This model, characterized by the absence of active bacterial infection and neutrophil consumption, has enabled us to dissect the host response to sepsis and to further prove a direct link between neutropenia and HSC function.
Finally, the critical importance of LPS-TLR4 signaling is validated by experiments in C3H/HeJ mice lacking TLR4 function. Previous in vitro experiments have suggested a role of TLR4 in HSC regulation.21 In our study, we demonstrated the critical role of TLR4 in regulating BM dynamics in vivo during sepsis by showing that functional TLR4 signaling is necessary to mediate HSPC expansion and reduction of myeloid progenitors. This observation is particularly relevant, as it demonstrates that the alterations of the HSC compartment and the resulting myelosuppresion are part of the host innate response to TLR4 activation by pathogens. Future studies will explore the contribution of direct versus indirect effects of TLR4 signaling on stem and progenitor cells, as several factors triggered by TLR4 signaling may converge on the hematopoietic stem cell and contribute to alter its normal activity.
In conclusion, our results establish a link between HSC regulation and host response in severe sepsis and demonstrate a novel role for TLR4 signaling in acute infections. These findings also suggest that prevention of the myeloid block at the HSC level could be a potential therapeutic target to complement the existing therapeutic strategies for the treatment of severe sepsis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr S. Spinola, Dr W. Clapp, and Dr M. Yoder (all from Indiana University School of Medicine) for helpful discussion. We thank Dr J. Hellman (MGH) for quality control test on LPS, and Regina Baldini (Massachusetts General Hospital) for bacterial cell-culture preparation.
This work was supported by the National Institutes of Health (grant no. R01-HL068256-05A2 to N.C.), the Shawalter grant (N.C.), and Shriners grant no. 8710 (to L.G.R.).
National Institutes of Health
Authorship
Contribution: S. Rodriguez designed experiments, performed animal studies, flow cytometric analysis and sorting, cell-culture experiments, and qRT-PCR, and wrote part of the manuscript; A.C. performed bacteremia experiments and flow cytometric analysis; B.G. designed experiments, performed animal studies, and performed and interpreted histopathologic analysis; C.M. performed bone marrow transplantations and endotoxemia studies; W.S.G. contributed to bone marrow transplantation experiments; L.F. performed endotoxemia studies and flow cytometric analysis; H.B. performed animal studies; H.H. analyzed histologic sections; D.M.D. performed cell sorting and flow cytometric analysis; C.A.K. performed statistical analysis; S. Rice performed cell sorting and 7-multicolor analysis; L.G.R. designed experiments and interpreted and supervised the bacteremia experiments; and N.C. designed the study and the experiments, analyzed the data, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nadia Carlesso, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut, Bldg R4, Rm 421, Indianapolis, IN 46202; e-mail: ncarless@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal