Abstract
Binding of factor VIII to membranes containing phosphatidyl-L-serine (Ptd-L-Ser) is mediated, in part, by a motif localized to the C2 domain. We evaluated a putative membrane-binding role of the C1 domain using an anti-C1 antibody fragment, KM33scFv, and factor VIII mutants with an altered KM33 epitope. We prepared a dual mutant Lys2092/Phe2093 → Ala/Ala (fVIIIYFP 2092/93) and 2 single mutants Lys2092 → Ala and Phe2093 → Ala. KM33scFv inhibited binding of fluorescein-labeled factor VIII to synthetic membranes and inhibited at least 95% of factor Xase activity. fVIIIYFP 2092/93 had 3-fold lower affinity for membranes containing 15% Ptd-L-Ser but more than 10-fold reduction in affinity for membranes with 4% Ptd-L-Ser. In a microtiter plate, KM33scFv was additive with an anti-C2 antibody for blocking binding to vesicles of 15% Ptd-L-Ser, whereas either antibody blocked binding to vesicles of 4% Ptd-L-Ser. KM33scFv inhibited binding to platelets and fVIIIYFP 2092/93 had reduced binding to A23187-stimulated platelets. fVIIIYFP 2092 exhibited normal activity at various Ptd-L-Ser concentrations, whereas fVIIIYFP 2093 showed a reduction of activity with Ptd-L-Ser less than 12%. fVIIIYFP 2092/93 had a greater reduction of activity than either single mutant. These results indicate that Lys 2092 and Phe 2093 are elements of a membrane-binding motif on the factor VIII C1 domain.
Introduction
Factor VIII functions as a cofactor in the membrane-bound intrinsic factor Xase complex. Together with the enzyme factor IXa, activated factor VIII binds to phosphatidyl-L-serine (Ptd-L-Ser)–containing membranes1,2 to form an enzyme complex that cleaves the zymogen factor X to factor Xa.3,4 Factor Xa is thereafter responsible for catalyzing prothrombin cleavage to thrombin.5 The importance of the factor Xase complex is illustrated by the disease hemophilia, in which a deficiency of factor VIII (hemophilia A) or factor IX (hemophilia B) leads to life-threatening bleeding. Despite the central importance of membrane binding, this aspect of factor VIII function remains poorly understood.
Factor VIII is synthesized as a single polypeptide chain containing 2351 amino acids (molecular weight, 280 kDa) and shows a domain structure of A1-a1-A2-a2-B-a3-A3-C1-C2, where a1, a2, and a3 are spacer regions that separate the domains from each other.6 Factor VIII is homologous to factor V in amino acid sequence and domain structure.7 The A domains are homologous with ceruloplasmin, the C domains with discoidin I, and with lactadherin,8,9 and the B domain is unique to each protein.10 The A domains mediate the dominant interactions with factor IXa and factor X in the factor Xase complex, whereas binding to Ptd-L-Ser–containing membranes is mediated predominantly by the C2 domain.11-15 The structure-function relationships of factor V resemble those of factor VIII in that the A domains mediate the dominant interactions with the enzyme and substrate and the C2 domain mediates the dominant membrane-binding interaction.16 However, factor V has a second membrane-interactive motif localized to the C1 domain.17,18 The present study investigates a second membrane-interactive motif of factor VIII, localized to the C1 domain analogous to the second membrane-interactive motif of factor V.
The central element of treatment for hemophilia A is infusing recombinant factor VIII or purified plasma factor VIII into deficient patients. However, such treatment can result in the production of specific antibodies that neutralize function of factor VIII in the circulation.19,20 Some inhibitory antibodies block membrane-interactive epitope(s) of factor VIII, underscoring the importance of membrane binding for normal function. Indeed, these antibodies have helped to identify membrane-interactive motifs of factor VIII.21 A novel inhibitory antibody derived from the circulating B lymphocytes of a hemophilia A patient22 and directed against the factor VIII C1 domain was essential for the current study.23
A model of membrane-bound factor VIII, based on electron crystallography studies, places the C1 domain opposite the C2 domain and away from the membrane.24 In contrast, the recently reported lipid-free factor VIII crystal structures provide the detailed tertiary structure of the C1 domain as well as an alternate orientation.25,26 These structures indicate that the C1 domain is oriented nearly parallel to the C2 domain, positioned favorably for interaction with a membrane. Amino acids Lys 2092 and Phe 2093 protrude from the tip of the C1 domain, homologous with the membrane-interactive amino acids at the tip of the factor V C1 domain (Figure 1B). Evidence for a possible membrane-binding function for the C1 domain arises because intact factor VIII or the factor VIII light chain binds with a higher affinity to phospholipid vesicles and activated platelets than C2 alone, suggesting a role of the A3-C1 domain in membrane binding.15,27,28 Moreover, a role for the C1 domain in platelet binding has been recently described.27 Thus, we wished to evaluate the possibility that the C1 domain contributes to membrane binding and evaluate a particular epitope and amino acids that may participate.
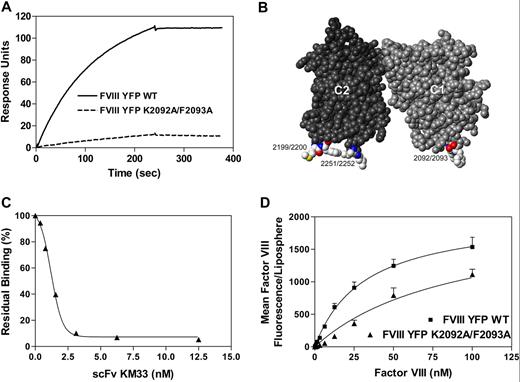
Identification of a factor VIII C1 domain epitope that participates in binding to phospholipid membranes. (A) Factor VIII residues K2092 to F2093 are critical for KM33 binding. KM33scFv (5 nM) was passed over fVIIIYFP or fVIIIYFP 2092/93 as described in detail in “Surface plasmon resonance.” Dissociation was initiated on replacement of ligand solution by buffer. (B) Space-filling display of the C1 and C2 domains of factor VIII crystal structure (PDB ID: 2r7e). The C2 domain is depicted in dark gray, whereas the C1 domain is medium gray. Mutated residues of the KM33scFv epitope on the C1 domain and the 4 membrane-interactive hydrophobic residues of the C2 domain are highlighted by coloring according to the CPK scheme. (C) KM33scFv inhibits membrane binding of factor VIII. Fluorescein-labeled factor VIII (3 nM) was incubated with KM33scFv (0-12.5 nM) before the addition of 15% Ptd-L-Ser–containing lipospheres. After 15 minutes, bound factor VIII was measured by flow cytometry. The result displayed represents the average of 2 experiments. (D) fVIIIYFP 2092/93 has reduced affinity for phospholipid membranes. fVIIIYFP (■) or fVIIIYFP 2092/93 (▲) was incubated with lipospheres containing 15% Ptd-L-Ser. After 15 minutes, bound factor VIII was determined by flow cytometry. Data represent the mean ± SEM of 3 experiments. KD values obtained from fitted curves were 31 nM ± 2 nM for fVIIIYFP and 91 nM ± 6 nM for factor fVIIIYFP 2092/93. The KD value for fVIIIYFP 2092/93 was obtained by assuming that the maximum phospholipid binding, at saturation, would be equivalent to wild-type factor VIII.
Identification of a factor VIII C1 domain epitope that participates in binding to phospholipid membranes. (A) Factor VIII residues K2092 to F2093 are critical for KM33 binding. KM33scFv (5 nM) was passed over fVIIIYFP or fVIIIYFP 2092/93 as described in detail in “Surface plasmon resonance.” Dissociation was initiated on replacement of ligand solution by buffer. (B) Space-filling display of the C1 and C2 domains of factor VIII crystal structure (PDB ID: 2r7e). The C2 domain is depicted in dark gray, whereas the C1 domain is medium gray. Mutated residues of the KM33scFv epitope on the C1 domain and the 4 membrane-interactive hydrophobic residues of the C2 domain are highlighted by coloring according to the CPK scheme. (C) KM33scFv inhibits membrane binding of factor VIII. Fluorescein-labeled factor VIII (3 nM) was incubated with KM33scFv (0-12.5 nM) before the addition of 15% Ptd-L-Ser–containing lipospheres. After 15 minutes, bound factor VIII was measured by flow cytometry. The result displayed represents the average of 2 experiments. (D) fVIIIYFP 2092/93 has reduced affinity for phospholipid membranes. fVIIIYFP (■) or fVIIIYFP 2092/93 (▲) was incubated with lipospheres containing 15% Ptd-L-Ser. After 15 minutes, bound factor VIII was determined by flow cytometry. Data represent the mean ± SEM of 3 experiments. KD values obtained from fitted curves were 31 nM ± 2 nM for fVIIIYFP and 91 nM ± 6 nM for factor fVIIIYFP 2092/93. The KD value for fVIIIYFP 2092/93 was obtained by assuming that the maximum phospholipid binding, at saturation, would be equivalent to wild-type factor VIII.
In the present study, we investigated the role of the factor VIII C1 domain in binding to synthetic phospholipid membranes and platelet membranes. To investigate this, we prepared a human single-chain variable domain antibody fragment (KM33scFv) against the hypothetical membrane-interactive C1 domain epitope.22,23 In addition, we prepared factor VIII mutants in which Lys 2092 and/or Phe 2093 within the epitope were changed to alanine. The results demonstrate that the C1 domain participates in binding to Ptd-L-Ser–containing membranes and that Lys 2092 and Phe 2093 participate in this interaction. In addition, these amino acids of the factor VIII C1 domain participate in binding to platelets to provide full cofactor activity of factor VIII.
Methods
Materials
L-α-Phosphatidylcholine (PC) from egg yolk, L-α-phosphatidylserine from bovine brain, L-α-phosphatidylethanolamine (PE) from egg yolk, and 1-oleoyl-2-(12-biotinyl(aminododecanoyl))-sn-glycero-3-phosphoethanolamine were from Avanti Polar Lipids. Fluorescein 5-maleimide was from Invitrogen. Pfu HotStart polymerase was from Stratagene. Oligonucleotide primers, dNTPs, expression vector pcDNA3.1 (+), DMRIE-C reagent, Geneticin G-418 sulfate, and trypsin were supplied by Invitrogen. Dulbecco modified Eagle medium/F12 medium was from Lonza. Fetal calf serum was from HyClone (Thermo Electron). ExtrAvidin peroxidase conjugate and o-phenylene diamine dihydrochloride tablet sets, thrombin receptor activating peptide (TRAP), SFLLRNPNDKYEPF, A23187, prostaglandin E1, and Optiprep were obtained from Sigma-Aldrich. Human α thrombin, human factor IXa, and human factor X were obtained from Enzyme Research Laboratories. Glass microspheres were from Duke Scientific.
Proteins
Single-chain variable antibody fragment KM33 (KM33scFv) was expressed in Escherichia coli strain XL1-Blue (deleted TG1) and purified by metal chelate chromatography (GE Healthcare) as described.22 Human full-length monoclonal antibody, BO2C11, was a generous gift from Prof Marc Jacquemin (Katholieke Universiteit, Leuven, Belgium). Human monoclonal antibody EL14 IgG4, directed against the C2 domain, was constructed and expressed as described.29
B-domain–deleted FVIII-YFP (fVIIIYFP) in pcDNA3.1(+) was constructed as described.30 The Lys2092Ala/Phe2093Ala mutation was introduced by Quik Change mutagenesis using primers (sense) 5′-ACCCAGGGTGCCCGTCAGGCGGCCTCCAGCCTCTACATCTCT-3′ and (antisense) 5′-AGAGATGTAGAGGCTGGAGGCCGCCTGACGGGCACCCTGGGT-3′. The separate mutations, Lys2092Ala and Phe2093Ala, were introduced using the primers (sense) 5′-CCCAGGGTGCCCGTCAGGCGTTCTCCAGCC-3′, (antisense) 5′-GATGTAGAGGCTGGAGAACGCCTGACGGGC-3′, (sense) 5′-GCCCGTCAGAAGGCCTCCAGCCTCTAC-3′, and (antisense) 5′-GTAGAGGCTGGAGGCCTTCTGACGGGC-3′. Coding regions of all constructs were verified by sequence analysis. Sequence reactions were performed using the BigDye Terminator Sequencing kit (Applied Biosystems). HEK293 cell lines, stably expressing recombinant protein, were produced as described.31 HEK293 cells were grown in Dulbecco modified Eagle medium-F12 medium supplemented with 10% fetal calf serum. Recombinant FVIII proteins were purified from conditioned medium by immunoaffinity chromatography using monoclonal antibody (mAb) CLB-CAg117 as previously described.30 Protein concentrations were determined by the method of Bradford.32 The factor VIII concentration was determined by an enzyme-linked immunosorbent assay essentially as described.33 FVIII activity was determined with a chromogenic assay according to the manufacturer's instructions (Chromogenix).
Fluorescein labeling of factor VIII
Factor VIII was labeled with fluorescein-maleimide as previously described.34 Protein concentration of factor VIII was determined using a Micro-BCA assay (Pierce Chemical) using bovine albumin as a standard. Factor VIII activity was determined using an activated partial thromboplastin time assay with factor VIII–deficient plasma, using normal plasma as a control.
Phospholipid vesicles
Large multilamellar phospholipid vesicles were prepared by evaporating chloroform from the desired phospholipids, resuspending the lipids in methylene chloride, and reevaporating 3 times under argon. Lipids were resuspended as vesicles by gently swirling Tris-buffered saline over the dried lipid, also under an argon stream. Small unilamellar phospholipid vesicles were prepared by sonication in a bath sonicator (Laboratory Supplies) until the suspension was visually clear.35 Vesicles were quick frozen in liquid nitrogen and stored at −80°C.
Liposphere preparation
Glass microspheres of 1.6 μM nominal diameter were cleaned, size restricted, and covered with a phospholipid bilayer as previously described.34 Briefly, microspheres were incubated with phospholipid vesicles while additional sonication was applied. Lipospheres were washed 4 times in 0.15 M NaCl, 0.04 M Tris-HCl, 0.1% defatted bovine albumin, 10 μM egg PC as sonicated vesicles, stored on ice, and used within 8 hours of synthesis.
Platelet isolation and activation
Human platelets were purified from whole blood from healthy donors as described.36 A 19-G butterfly needle and a 2-syringe technique were used. Whole blood was drawn into acid citrate dextrose containing 10 mM prostaglandin E1. Platelet-rich plasma was isolated by centrifugation at 200g for 15 minutes at room temperature. Platelet-rich plasma was next layered onto a discontinuous density gradient consisting of 1.5 mL Optiprep 1.320 overlaid with 625 μL Optiprep diluted into 2 mL dH2O and 2 mL Tyrode piperazine-N, N-bis[2-ethanesulfonic acid] buffer (15 mmol/L piperazine-N, N-bis[2-ethanesulfonic acid], pH 6.8, 3.3 mmol/L NaH2PO4, 138 mmol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2, 5.5 mmol/L dextrose containing 0.2% [wt/vol] bovine serum albumin). A band of concentrated platelets was retrieved from the interface after centrifugation for 30 minutes at 300g at room temperature. Platelets were diluted 1:1 with Tyrode N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (20 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 3.3 mmol/L NaH2PO4, 138 mmol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2, 5.5 mmol/L dextrose) 0.2% bovine serum albumin and counted using a hemocytometer. Platelet experiments were performed at room temperature within 8 hours of venipuncture.
Flow cytometry measurements of factor VIII binding
Factor VIII binding to lipospheres was measured using a BD Biosciences FACSCalibur flow cytometer.34 Factor VIII was incubated with 5 × 106 lipospheres/mL in 0.15 M NaCl, 0.04 M Tris-HCl, 0.1% defatted bovine albumin, 1.5 mM CaCl2, pH 7.8, for 15 minutes at room temperature before evaluating the binding of factor VIII to lipospheres. For measurements in the presence of KM33scFv, 3 nM FVIII was incubated with KM33scFv for 30 minutes at room temperature before lipospheres were added. Values from fVIIIYFP or fVIIIYFP 2092/93 were corrected for the quantity of fluorescence from fVIIIYFP or fVIIIYFP 2092/93 (0-100 nM) incubated with lipospheres containing 100% PC membranes. Data acquisition was triggered by forward light scatter with all photomultipliers in the log mode. Noise was reduced during analysis by eliminating events with forward and side scatter values different from those characteristic of the lipospheres. Geometric mean log fluorescence was converted to linear fluorescence for values depicted in Figure 1. In platelet-binding experiments, platelets were activated with 10 μM TRAP or A23187 for 10 minutes at room temperature; 10 nM factor VIII was incubated with 107 activated platelets/mL for 10 minutes at room temperature and diluted 1:10 into Tyrode N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, 0.2% bovine serum albumin immediately before evaluating factor VIII binding with flow cytometry.
Microtiter assay of phospholipid vesicle binding
For nonequilibrium binding assays, microtiter wells were coated with mAb ESH8 (5 μg/mL) and incubated overnight at 4°C. Next, wells were blocked with 0.15 M NaCl, 0.04 M Tris-HCl plus 3% defatted bovine albumin for 2 hours at 37°C. After blocking, wells were incubated with factor VIII for 1.5 hours at room temperature. Next, KM33scFv, BO2C11 or KM33scFv plus BO2C11 was added to the wells, and this mixture was incubated for 1.5 hours at room temperature. Phospholipid vesicles containing 1% biotin-PE were then incubated 2 hours at room temperature with the immobilized factor VIII. Streptavidin-horseradish peroxidase (HRP), 0.25 μM, was incubated with the vesicles. After washing the plates 3 times, the substrate o-phenylene diamine dihydrochloride was added. After 15 minutes of incubation at room temperature, bound phospholipid vesicles were determined by measuring the OD at 450 nm. All wells were washed 3 times between each incubation step with 0.15 M NaCl, 0.04 M Tris-HCl, pH 7.8.
Factor Xase assay
Factor Xase activity was measured with a 2-step amidolytic substrate assay. Sonicated phospholipid vesicles were mixed with 1 U/mL factor VIII, 4 nM factor IXa, and 200 nM factor X in 0.15 M NaCl, 0.04 M Tris-HCl plus 0.2% (wt/vol) bovine serum albumin, pH 7.8. Phospholipid vesicles had compositions with the indicated Ptd-L-Ser content, 20% PE, and the balance as PC. The reaction was started by adding Ca2+ and thrombin at a final concentration of 1.5 mM and 0.8 U/mL, respectively. After 5 minutes of incubation at 25°C, the reaction was stopped by diluting the mixture 1:0.75 with 16 mM ethylenediaminetetraacetic acid. The factor Xa activity was determined immediately in a thermostatted kinetic microtiter plate reader (Molecular Devices) at 25°C using S-2765. Absorbance values were converted into molar concentrations using a standard curve of pure factor Xa. In platelet experiments with KM33scFv, 0.5 × 108 platelets/mL were used. In experiments with fVIIIYFP and fVIIIYFP 2092/93, 0.2 to 0.25 × 108 platelets/mL were used. Platelets were activated with 10 μM TRAP or A23187 before adding coagulation factors. Reaction was performed as described in this section for phospholipid vesicles.
Surface plasmon resonance
Surface plasmon resonance analysis was performed using a BIAcore3000 biosensor system (Biacore AB). Human monoclonal antibody EL14 was covalently coupled (53 fmol/mm2) to the dextran surface of an activated CM5-sensor chip via primary amino groups, using the amine-coupling kit as prescribed by the supplier. Next, fVIIIYFP and fVIIIYFP 2092/93 were loaded on EL14 to a density of 5 fmol/mm2. Factor VIII–loaded chips were used for KM33scFv binding studies. For KM33scFv, association and dissociation were assessed in 150 mM NaCl, 5 mM CaCl2, 0.005% (vol/vol) Tween 20, and 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.4) at a flow rate of 20 μL/min for 4 minutes at 25°C. Association and dissociation curves were corrected for nonspecific binding, which was less than 5% of the total signal.
Results
Anti-C1 monoclonal antibody inhibits binding of factor VIII to phospholipid membranes
To confirm that the epitope of KM33scFv includes the amino acids at the lower tip of the factor VIII C1 domain, we prepared a factor VIII mutant (fVIIIYFP 2092/93) in which residues K2092 and F2093 were changed to alanines. Mutations were prepared within a construct where the factor VIII B domain was replaced by yellow fluorescent protein to facilitate evaluation of membrane binding (fVIIIYFP).30 Binding of KM33scFv to fVIIIYFP 2092/93 was greatly reduced compared with fVIIIYFP, confirming the importance of K2092 and F2093 in the KM33scFv epitope (Figure 1A). These residues protrude from a surface that is oriented at the same direction as the established lipid-binding loops of the C2 domain (Figure 1B). To determine whether the C1 domain epitope of KM33 (Figure 1A-B) is involved in membrane binding, we performed membrane-binding experiments in the presence of KM33scFv. The effect of KM33scFv was investigated by flow cytometry using membranes supported by glass microspheres (lipospheres) as a model to investigate the binding of factor VIII (Figure 1C).34 KM33scFv inhibited approximately 95% of the binding of factor VIII to membranes containing 15% Ptd-L-Ser. These data suggest that the C1 domain of factor VIII is involved in binding to phospholipid membranes.
Role of C1 amino acids Lys 2092/Phe 2093 in membrane binding
Binding of fVIIIYFP and fVIIIYFP 2092/93 to lipospheres was determined by flow cytometry (Figure 1D). The results indicated that fVIIIYFP 2092/93 has reduced affinity for membranes containing 15% Ptd-L-Ser compared with fVIIIYFP. The apparent dissociation constants were 31 nM plus or minus 2 nM and 91 nM plus or minus 6 nM for fVIIIYFP and fVIIIYFP 2092/93, respectively. Thus, mutation of Lys 2092 and Phe 2093 leads to an approximately 3-fold reduction in affinity for membrane-binding sites. The apparent KD of fVIIIYFP is somewhat higher than wild-type recombinant factor VIII1,34 as discussed in “Discussion.” We also investigated the binding of fVIIIYFP and fVIIIYFP 2092/93 to lipospheres containing 4% Ptd-L-Ser membranes. However, the binding curves of fVIIIYFP 2092/93 did not reach a clear inflection point at achievable protein concentrations (data not shown). These results suggest that Lys 2092 and/or Phe 2093 of the factor VIII C1 domain are involved in binding to phospholipid membranes.
The KM33 C1 domain epitope is required for full cofactor function of factor VIII
To evaluate the functional importance of the KM33 epitope, we performed a factor Xase assay in the presence and absence of KM33scFv. The generation of factor Xa was measured in the presence of various concentrations of phospholipid vesicles containing 15% or 4% Ptd-L-Ser (Figure 2A-B). The half-maximal phospholipid concentrations were 0.26 μM and 4.0 μM for phospholipid vesicles containing 15% or 4% Ptd-L-Ser, respectively. Factor VIII bound to KM33scFv was unable to support factor Xase activity to any appreciable extent in the presence of 4% Ptd-L-Ser–containing phospholipid vesicles. Similar results were obtained with phospholipid vesicles containing 15% Ptd-L-Ser. In the presence of KM33scFv, a higher concentration of vesicles was required and approximately 5% factor Xase activity remained. These results indicate that blockade of the KM33 epitope diminishes the phospholipid-dependent function of factor VIII, leading to approximately 95% reduction of activity.
Function of KM33 epitope in activity of factor VIII. KM33scFv inhibited activity of factor VIII on vesicles with 15% Ptd-L-Ser (A) and on vesicles with 4% Ptd-L-Ser (B). Factor VIII (1 U/mL) in the absence (■) or presence (▲) of excess KM33scFv was added to various concentrations of phospholipid vesicles. Factor VIII was preincubated with KM33scFv for 30 minutes at room temperature. Displayed values represent the mean ± SEM for 3 separate experiments, each performed in duplicate. (C-D) FVIIIYFP K2092A/F2093A had diminished apparent affinity for phospholipid vesicles. fVIIIYFP (■) or fVIIIYFP 2092/93 (▲) (1 U/mL) was added to various concentrations of phospholipid vesicles containing 15% Ptd-L-Ser (C) or 4% Ptd-L-Ser (D), and activity was measured as described for panels A and B. Each point represents the mean value ± SEM of 3 experiments. Curves were fitted to a single-site binding model, assuming that vesicles offered a single class of binding sites, and fitted with nonlinear least squares curve fitting.
Function of KM33 epitope in activity of factor VIII. KM33scFv inhibited activity of factor VIII on vesicles with 15% Ptd-L-Ser (A) and on vesicles with 4% Ptd-L-Ser (B). Factor VIII (1 U/mL) in the absence (■) or presence (▲) of excess KM33scFv was added to various concentrations of phospholipid vesicles. Factor VIII was preincubated with KM33scFv for 30 minutes at room temperature. Displayed values represent the mean ± SEM for 3 separate experiments, each performed in duplicate. (C-D) FVIIIYFP K2092A/F2093A had diminished apparent affinity for phospholipid vesicles. fVIIIYFP (■) or fVIIIYFP 2092/93 (▲) (1 U/mL) was added to various concentrations of phospholipid vesicles containing 15% Ptd-L-Ser (C) or 4% Ptd-L-Ser (D), and activity was measured as described for panels A and B. Each point represents the mean value ± SEM of 3 experiments. Curves were fitted to a single-site binding model, assuming that vesicles offered a single class of binding sites, and fitted with nonlinear least squares curve fitting.
We next investigated whether Lys 2092 and/or Phe 2093 are necessary for the cofactor function of factor VIII in the factor Xase complex (Figure 2C-D). The results demonstrate that the apparent affinity of fVIIIYFP 2092/93 for phospholipid vesicles with 15% Ptd-L-Ser was slightly decreased compared with wild-type factor VIII and fVIIIYFP. Apparent half-maximal phospholipid concentrations were 0.22 μM and 0.88 μM for fVIIIYFP and fVIIIYFP 2092/93, respectively. These implied dissociation constants are similar to the equilibrium-binding values, where we found a 3-fold reduction in affinity of the mutant for 15% Ptd-L-Ser membranes. However, the maximum activity of fVIIIYFP 2092/93 was at least as high as fVIIIYFP when saturating vesicles were present, indicating that KM33scFv inhibits activity to a greater extent than mutation of Lys 2092/Phe 2093.
When the Ptd-L-Ser content was reduced to 4% (Figure 2D), the apparent affinity and the cofactor function of fVIIIYFP 2092/93 were greatly impaired. Assuming the same equilibrium plateau as wild-type factor VIII or fVIIIYFP, the affinity of the mutant was decreased approximately 40-fold. Additional results indicated that saturation of factor Xase complex supported by fVIIIYFP occurred at a phospholipid concentration of 32 μM. In contrast, the fVIIIYFP 2092/93 did not exhibit a clear inflection point at phospholipid concentration of 128 μM (data not shown). These results confirm that the affinity of factor VIII for phospholipid membranes is reduced when Lys 2092 and Phe 2093 are replaced by alanines.
Factor VIII binding to phospholipid vesicles in the presence of monoclonal antibodies against the C1 and the C2 domain
To gain additional insights into the contributions of the C1 domain versus the C2 domain to binding phospholipid vesicles, we developed a nonequilibrium binding assay. The binding of sonicated vesicles to immobilized factor VIII was investigated in the presence of KM33scFv and/or mAb BO2C11 (Figure 3). BO2C11 is a human mAb originally derived from a hemophilia A patient. Epitope mapping of this antibody indicated that BO2C11 interacts with residues located between amino acids residues 2170 to 2216 and 2302 to 2332 of the carboxy-terminal region of the C2 domain of factor VIII.37 A cocrystal of B02C11 and the factor VIII C2 domain indicates that B02C11 engages the membrane-interactive hydrophobic amino acids of the C2 domain (Figure 1A).21 Factor VIII was immobilized via monoclonal antibody ESH8 and then incubated with KM33scFv, BO2C11, or both antibodies together. Next, the immobilized factor VIII-antibody complexes were incubated with phospholipid vesicles containing biotin-PE. After washing, the quantity of bound phospholipid vesicles was determined with ExtraVidin-HRP reagents (“Microtiter assay of phospholipid vesicle binding”).
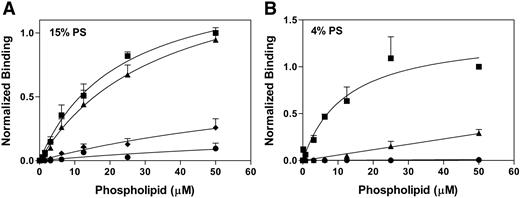
Effect of KM33scFv and/or anti-C2 domain mAb BO2C11 on vesicles binding to immobilized factor VIII. Immobilized factor VIII was incubated with a molar excess of KM33scFv and/or BO2C11. Various concentrations of vesicles containing 1% biotin-PE and either 15% Ptd-L-Ser (A) or 4% Ptd-L-Ser (B) were incubated with the immobilized complex of factor VIII with inhibitory antibodies. Data show factor VIII without inhibitory antibodies (■), in the presence of KM33scFv (▲), in the presence of BO2C11 (♦), or both KM33scFv BO2C11 (●). Each point represents the mean value ± SEM of 4 (A) or 3 (B) experiments.
Effect of KM33scFv and/or anti-C2 domain mAb BO2C11 on vesicles binding to immobilized factor VIII. Immobilized factor VIII was incubated with a molar excess of KM33scFv and/or BO2C11. Various concentrations of vesicles containing 1% biotin-PE and either 15% Ptd-L-Ser (A) or 4% Ptd-L-Ser (B) were incubated with the immobilized complex of factor VIII with inhibitory antibodies. Data show factor VIII without inhibitory antibodies (■), in the presence of KM33scFv (▲), in the presence of BO2C11 (♦), or both KM33scFv BO2C11 (●). Each point represents the mean value ± SEM of 4 (A) or 3 (B) experiments.
As expected, BO2C11 inhibits binding of factor VIII to phospholipid vesicles (Figure 3). Binding to phospholipid vesicles with 4% Ptd-L-Ser is completely inhibited by BO2C11 (Figure 3B), whereas binding to vesicles containing 15% Ptd-L-Ser was not inhibited completely (Figure 3A). This implies that a domain besides C2 contributes to binding phospholipid membranes. The binding of factor VIII to phospholipid vesicles was also inhibited in the presence of KM33scFv. The degree of inhibition was dependent on the amount of Ptd-L-Ser present in the phospholipid vesicles. KM33scFv had only a small effect on binding of vesicles containing 15% Ptd-L-Ser to factor VIII (Figure 3A). In contrast, binding 4%Ptd-L-Ser vesicles was inhibited approximately 75% (Figure 3B) at a 50-μM phospholipid concentration, with a larger apparent impact on the implied affinity. The combination of B02C11 and KM33scFv inhibited more than 85% of measured binding to vesicles with 15% Ptd-L-Ser. These results confirm that the C1 domain contributes to membrane binding and that the requirement of the C1 domain is related to the Ptd-L-Ser content.
The C1 domain region 2092/2093 is involved in the interaction with the platelet membrane
To assess the role of this region in interaction with the platelet membrane, we performed binding studies by flow cytometry and factor Xase assays with human platelets from healthy blood donors (Figure 4). Platelets were purified using a density gradient and activated with either TRAP or A23187 to induce limited or complete Ptd-L-Ser exposure.38
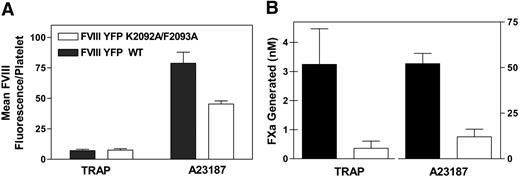
Effect of KM33scFv on factor VIII binding to and function on platelets. (A) Fluorescein maleimide–labeled factor VIII was preincubated with KM33scFv for 30 minutes at room temperature. Platelets were stimulated with 10 μM TRAP or A23187 for 10 minutes and then incubated with factor VIII-fluorescein (10 nM) at room temperature. Bound factor VIII was measured after 10 minutes by flow cytometry. Values were corrected for the quantity of fluorescence from factor VIII ± KM33scFv in the presence of resting platelets (21-33 fluorescence units). Bars represent the mean ± SEM of 3 experiments. (B) Factor VIII (5 U/mL) was incubated with KM33scFv for 30 minutes at room temperature. Factor VIII-antibody complex was added to platelets activated for 10 minutes at room temperature with either 10 μM TRAP or A23187. Factor IXa, factor X, and Ca2+ were added to start the reaction. After 5 minutes, the reaction was stopped with ethylenediaminetetraacetic acid, and the generation of factor Xa was measured by monitoring the rate of cleavage of chromogenic substrate S2765. Values represent the average of 3 experiments and are normalized for comparison of the extent of inhibition. Maximum activity ranged from 8 to 18 nM FXa generated for TRAP-stimulated platelets and from 60 to 157 nM FXa generated for A23187-stimulated platelets.
Effect of KM33scFv on factor VIII binding to and function on platelets. (A) Fluorescein maleimide–labeled factor VIII was preincubated with KM33scFv for 30 minutes at room temperature. Platelets were stimulated with 10 μM TRAP or A23187 for 10 minutes and then incubated with factor VIII-fluorescein (10 nM) at room temperature. Bound factor VIII was measured after 10 minutes by flow cytometry. Values were corrected for the quantity of fluorescence from factor VIII ± KM33scFv in the presence of resting platelets (21-33 fluorescence units). Bars represent the mean ± SEM of 3 experiments. (B) Factor VIII (5 U/mL) was incubated with KM33scFv for 30 minutes at room temperature. Factor VIII-antibody complex was added to platelets activated for 10 minutes at room temperature with either 10 μM TRAP or A23187. Factor IXa, factor X, and Ca2+ were added to start the reaction. After 5 minutes, the reaction was stopped with ethylenediaminetetraacetic acid, and the generation of factor Xa was measured by monitoring the rate of cleavage of chromogenic substrate S2765. Values represent the average of 3 experiments and are normalized for comparison of the extent of inhibition. Maximum activity ranged from 8 to 18 nM FXa generated for TRAP-stimulated platelets and from 60 to 157 nM FXa generated for A23187-stimulated platelets.
KM33scFv inhibited 80% to 95% of binding of factor VIII to platelets stimulated with either TRAP or A23187. KM33scFv inhibited the platelet-supported activity of factor VIII more than 95% for both agonists. These results confirm that the C1 domain of factor VIII is required for normal platelet binding as well as full function of factor VIII.
We next investigated the binding of fVIIIYFP and fVIIIYFP 2092/93 to platelets by flow cytometry (Figure 5A). fVIIIYFP 2092/93 showed reduced binding to platelets stimulated with A23187, as predicted. However, the binding of fVIIIYFP 2092/93 to TRAP-stimulated platelets was comparable with fVIIIYFP. These results imply that the role of Lys 2092 and/or Phe 2093 in binding to TRAP-stimulated platelets differs from the role in binding to membranes with 4% Ptd-L-Ser and from the role in binding to platelets with complete Ptd-L-Ser exposure. A factor Xase assay was performed to assess the role of these amino acid residues in the platelet-dependent factor Xase complex (Figure 5B). Function of fVIIIYFP 2092/93 was decreased more than 80% compared with fVIIIYFP when platelets were stimulated with either TRAP or A23187. These results confirm that amino acids Lys 2092 and/or Phe 2093 are necessary for normal function on the platelet membrane.
Function of Lys 2092 and Phe 2093 in binding to platelets and platelet-dependent activity. (A) fVIIIYFP (solid columns) or fVIIIYFP 2092/93 (open columns) (10 nM) were incubated with platelets stimulated with TRAP or A23187. After 10 minutes of incubation at room temperature, bound factor VIII was determined by flow cytometry. Values were corrected for the quantity of fluorescence from factor VIII in the presence of resting platelets (7-11 fluorescence units). Bars represent the mean ± SEM of at least 4 experiments. (B) Platelets were activated with TRAP or A23187 for 10 minutes at room temperature and added to factor VIII, factor IXa, factor X, and Ca2+. After 5 minutes, the reaction was stopped by adding 16 mM ethylenediaminetetraacetic acid. The generation of factor Xa was measured by monitoring the rate of cleavage of chromogenic substrate S2765. Solid columns represent fVIIIYFP; open columns, fVIIIYFP 2092/93. Data represent the mean ± SEM for 4 experiments. Maximum activity for TRAP-stimulated platelets ranged from 0.5 to 5.5 nM (fVIIIYFP) and 0 to 1.0 nM (fVIIIYFP 2092/93) FXa generated and from 37 to 61 nM (fVIIIYFP) and 9 to 21 nM (fVIIIYFP 2092/93) FXa generated for A23187-stimulated platelets.
Function of Lys 2092 and Phe 2093 in binding to platelets and platelet-dependent activity. (A) fVIIIYFP (solid columns) or fVIIIYFP 2092/93 (open columns) (10 nM) were incubated with platelets stimulated with TRAP or A23187. After 10 minutes of incubation at room temperature, bound factor VIII was determined by flow cytometry. Values were corrected for the quantity of fluorescence from factor VIII in the presence of resting platelets (7-11 fluorescence units). Bars represent the mean ± SEM of at least 4 experiments. (B) Platelets were activated with TRAP or A23187 for 10 minutes at room temperature and added to factor VIII, factor IXa, factor X, and Ca2+. After 5 minutes, the reaction was stopped by adding 16 mM ethylenediaminetetraacetic acid. The generation of factor Xa was measured by monitoring the rate of cleavage of chromogenic substrate S2765. Solid columns represent fVIIIYFP; open columns, fVIIIYFP 2092/93. Data represent the mean ± SEM for 4 experiments. Maximum activity for TRAP-stimulated platelets ranged from 0.5 to 5.5 nM (fVIIIYFP) and 0 to 1.0 nM (fVIIIYFP 2092/93) FXa generated and from 37 to 61 nM (fVIIIYFP) and 9 to 21 nM (fVIIIYFP 2092/93) FXa generated for A23187-stimulated platelets.
Contributions of Lys 2092 and Phe 2093 in relationship to membrane Ptd-L-Ser content
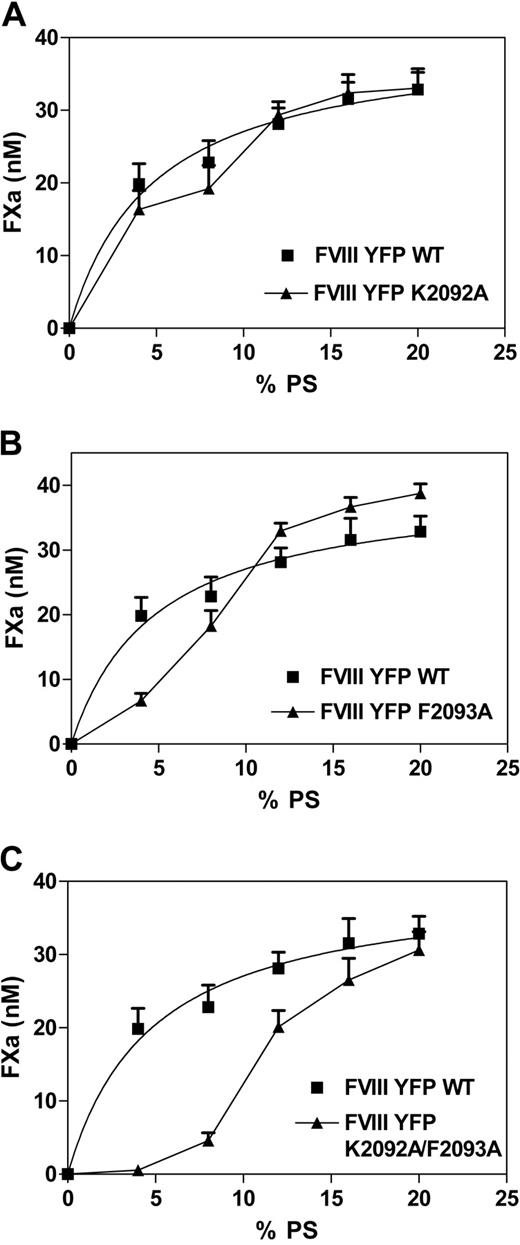
To assess the distinct contribution of residues 2092 and 2093, single point mutations of fVIIIYFP were prepared. Mutants were evaluated in a factor Xase assay, where activity was measured as a function of the Ptd-L-Ser content of vesicles (Figure 6). Function of fVIIIYFP 2092 was only slightly reduced compared with fVIIIYFP on vesicles with less than 12% Ptd-L-Ser (Figure 6A) and was equivalent for vesicles with higher Ptd-L-Ser content. In contrast, activity of fVIIIYFP 2093 (Figure 6B) was markedly reduced for vesicles with 4% Ptd-L-Ser and moderately reduced for vesicles with 8% Ptd-L-Ser. Activity was at least as high as for vesicles with at least 12% Ptd-L-Ser. As predicted from results in Figure 2, activity of fVIIIYFP 2092/93 was reduced for vesicles with Ptd-L-Ser content less than 20% (Figure 6C). The extent of activity decrease was greater than with either isolated point mutation. These results indicate that Phe 2093 makes a larger contribution than Lys 2092 in binding to membranes with Ptd-L-Ser content less than 12%. However, both residues appear to contribute to membrane binding because activity of fVIIIYFP 2092/93 is lower than activity for either single mutant.
Cofactor activity of mutants lacking Lys 2092 and/or Phe 2093 as a function of Ptd-L-Ser content in phospholipid vesicles. (A) Activity of fVIIIYFP (■) was compared with fVIIIYFP 2092 (▲) for vesicles with Ptd-L-Ser content ranging from 0% to 20%. (B) fVIIIYFP 2093 (▲) was compared with fVIIIYFP (■). (C) fVIIIYFP 2092/2093 (▲) was compared with fVIIIYFP (■). Results indicate that fVIIIYFP 2093 has intermediate impairment when Ptd-L-Ser content is less than 12%, whereas fVIIIYFP 2092 has activity approaching fVIIIYFP. The concentration of factor VIII was 1 U/mL, and vesicle composition was 0% to 20% Ptd-L-Ser, 20% PE, with the balance as PC. Assays were performed as for Figure 2. Each point represents the mean ± SEM for 3 experiments, each performed in duplicate. Data and curve for fVIIIYFP are displayed in panels A to C.
Cofactor activity of mutants lacking Lys 2092 and/or Phe 2093 as a function of Ptd-L-Ser content in phospholipid vesicles. (A) Activity of fVIIIYFP (■) was compared with fVIIIYFP 2092 (▲) for vesicles with Ptd-L-Ser content ranging from 0% to 20%. (B) fVIIIYFP 2093 (▲) was compared with fVIIIYFP (■). (C) fVIIIYFP 2092/2093 (▲) was compared with fVIIIYFP (■). Results indicate that fVIIIYFP 2093 has intermediate impairment when Ptd-L-Ser content is less than 12%, whereas fVIIIYFP 2092 has activity approaching fVIIIYFP. The concentration of factor VIII was 1 U/mL, and vesicle composition was 0% to 20% Ptd-L-Ser, 20% PE, with the balance as PC. Assays were performed as for Figure 2. Each point represents the mean ± SEM for 3 experiments, each performed in duplicate. Data and curve for fVIIIYFP are displayed in panels A to C.
Discussion
This study demonstrates that the factor VIII C1 domain is a component of the phospholipid membrane-binding mechanism. Amino acids Lys 2092 and Phe 2093, at the tip of the C1 domain, participate in the membrane binding, enhancing the function of factor VIII. The membrane-binding contribution of the factor VIII C1 domain is large for membranes with low Ptd-L-Ser content but modest for membranes with 15% Ptd-L-Ser. The factor VIII C1 domain also participates in binding to platelets where engagement of the C1 domain is necessary for full cofactor function.
Our findings are consistent with several prior reports. A preliminary report with antibody LE2E9, directed against the C1 domain, showed impaired membrane binding, thus suggesting that the C1 domain participates in binding to phospholipid vesicles.39 Experiments with the C1C2 domain fragment of factor VIII versus the C2 domain fragment indicated that the C1 domain contributes to platelet binding.27 Our results both confirm and extend these platelet studies by identifying membrane-interactive amino acids within the C1 domain and the role in binding platelets with maximal versus partial Ptd-L-Ser exposure. In addition, our study demonstrates the functional importance of the C1 domain for the platelet-supported factor Xase complex. As previously reported, we confirmed that the C2 domain contains the major membrane-interactive motif with the C1 domain, providing additional membrane-binding affinity.14,40,41
Our results with the factor VIII C1 domain parallel studies with the factor V C1 domain. For factor V, residues of both C domains have been identified as membrane-binding residues. Tyr 1956 and Leu 1957 of the factor V C1 domain, homologous with Lys 2092 and Phe 2093 of factor VIII, are involved in these interactions.17,18 A factor V mutant in which these residues were replaced by alanine had impaired binding to membranes with 5% Ptd-L-Ser but near-normal affinity to membranes with 25% Ptd-L-Ser.17
We developed a new assay for measuring phospholipid vesicle binding (Figure 3). In this assay, factor VIII is immobilized by monoclonal antibody ESH8, and binding of biotin-labeled vesicles is detected with avidin-HRP. Previous studies have shown that ESH8 is a minimally interfering tag to investigate membrane binding.27,42,43 The value of the assay is that it appears to report lower affinity binding interactions that are difficult to observe with our solution phase, quasi-equilibrium assays.34 The time frame for the prior assays emphasizes that they predominantly measure the more rapid phases of membrane association and dissociation. The high phospholipid concentrations, longer incubation times, and multiple wash steps in the new assay predict that the new assay allows time for completion of the slower association step and detects binding that has not yet completed the slower dissociation step.44 It is also possible that proximity of phospholipid vesicles to the microtiter well surface enhances the binding interaction with factor VIII.
Identification of Lys 2092 and Phe 2093 as membrane-interactive residues confirms that the overall orientation of factor VIII to a phospholipid membrane is similar to that predicted by the recent crystal structures of factor VIII.25,26 The orientation of the Lys 2092 side chain varies modestly between the 2 structures. However, crystal structures at this level of resolution (4 and 3.8 Å) cannot precisely define the orientation of side chains, and either structure is consistent with exposure of Lys 2092 to mAb KM33 and to a phospholipid membrane. Lys 2092 and Phe 2093 reside along an imaginary line drawn through the previously identified pairs of hydrophobic, membrane interactive amino acids of the factor VIII C2 domain, presumably in a plane that corresponds to the interfacial region of a phospholipid bilayer (Figure 1B). The experimental data do not establish the angle of elevation of the C1C2 domains about the line of membrane engagement. Additional experiments, identifying membrane-interactive residues at a distance from the line of engagement, will be necessary to determine the plane of engagement.
KM33scFv decreased membrane affinity and factor VIII activity more than mutation of Lys 2092 and Phe 2093 to alanines. This difference implies either that additional amino acids of the C1 domain participate in membrane interaction or that steric hindrance of the preferred orientation of the C1 domain leads to further diminution of membrane affinity. We are currently testing the hypothesis that additional C1 domain amino acids participate in membrane binding.
The C1 domain apparently has greater importance for engagement of membranes with 4% Ptd-L-Ser versus 15% Ptd-L-Ser. This difference parallels data reported for factor V, when homologous mutations were made in the C1 domain.17 These data underscore prior results indicating that factor VIII has 2 distinct modes of membrane binding.2 When the membrane content of Ptd-L-Ser is less than or equal to 8%, binding is dependent on stereoselective interaction with the head group of Ptd-L-Ser. When the Ptd-L-Ser content is more than or equal to 15%, the interaction is not specific for Ptd-L-Ser, with other negatively charged phospholipids serving to support high affinity binding. The results from our experiments indicate that the different mechanism uses different facets of factor VIII, or at least that different amino acids have substantially different importance for membranes with a high content of negatively charged lipids.
The total contribution of the Lys 2092/Phe 2093 is approximately 3-fold change in affinity, or approximately 0.8 kcal/mol, for membranes with 15% Ptd-L-Ser. This is a smaller contribution than would be predicted for loss of immersion of Phe in a hydrophobic environment or loss of a salt bridge between Lys and a negatively charged phosphatidylserine moiety, and we have considered 3 possible explanations. First, the relatively small loss of binding affinity may be explained by the difference between engagement of the K2093/F2093 motif and an alternative membrane-interactive motif, presumably on the C1 domain. Studies with the isolated factor VIII C2 domain suggest that the alternate membrane-interactive motif is unlikely to reside there (V. N. Novakovic and G.E.G., unpublished observations, September 2008). Second, a possible explanation for the relatively small loss of binding affinity results from an unfavorable conformational change induced by membrane binding. The change could be within the factor VIII C2 or C1 domain, or a change in the orientation of the C2 domain.25 A third possibility is that engagement of the Lys 2092/Phe 2093 motif with a phospholipid membrane may alter the local configuration of the phospholipid membrane in a manner that makes engagement of the C2 domain less favorable. It appears possible that the contribution of the Lys 2092/Phe 2093 motif approaches that predicted by a model in which Phe 2093 provides a major hydrophobic interaction. It is possible that the Lys 2092 charge helps to orient factor VIII for favorable membrane interaction during approach to the membrane and/or that Lys 2092 participates in the binding affinity via formation of hydrogen bond(s).
We note that the affinity of fVIIIYFP for phospholipid membranes is approximately 10-fold lower than wild-type factor VIII.1 This implies that the YFP domain has either a partial steric hindrance of a phospholipid membrane or that the YFP domain subtly modifies the factor VIII structure in such a way that phospholipid binding is altered. We do not think that this modest difference inhibits interpretation of our data. First, fVIIIYFP was used as controls for all binding experiments with fVIIIYFP 2092/93. fVIIIYFP was also used as the control for functional studies. Under these circumstances, the membrane affinity is unlikely to be altered by YFP because YFP is removed when thrombin activates factor VIII.30,45 Indeed, the implied affinity of fVIIIYFP for vesicles under these circumstances is similar to recombinant wild-type factor VIII (Figure 2).
An interesting result found in our study is that the binding of fVIIIYFP 2092/93 to TRAP-stimulated platelets is comparable with fVIIIYFP binding. This differs from binding of fVIIIYFP 2092/93 to A23187 stimulated platelets, which was reduced, compared with fVIIIYFP binding. Platelets stimulated by A23187 concentrations more than 1.0 μM have Ptd-L-Ser content of 10% to 15% in the external membrane.38 Thus, the Ptd-L-Ser content of A23187-stimulated platelets appears to reasonably explain the altered binding and function of fVIIIYFP 2092/93. The Ptd-L-Ser content on the surface of platelets stimulated by thrombin or TRAP is considerably lower,38 possibly in the range of 4% Ptd-L-Ser, as the vesicles used in this study. Thus, the equivalent binding of fVIIIYFP 2092/93 does not appear to be well explained, based on membrane Ptd-L-Ser content. This raised the question as to whether an unidentified component of platelet membranes is involved in binding to factor VIII. The mechanism behind the specific interaction of factor VIII and the platelet membrane and the components involved in this interaction remains a target for further investigation.
This report indicates that protruding amino acids at the tip of the factor VIII C1 domain participate in binding to Ptd-L-Ser–containing membranes and that engagement of these residues with the activated platelet membrane is essential for full activity of factor VIII. These findings pave the way for studies that will more fully define the facet(s) of the C1 domain that engage the membrane bilayer and determine whether the stereoselective Ptd-L-Ser recognition capability resides within the C1 domain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Marc Jacquemin for providing the BO2C11 antibody, Maartje van den Biggelaar for providing the factor VIII YFP construct, and Valerie Novakovic and Jialan Shi for assistance with liposphere and platelet assays.
Authorship
Contribution: H.M. designed and performed experiments in consultation with A.B.M., K.M., and G.E.G.; H.M. and G.E.G. drafted the manuscript; D.B.C. developed the microtiter vesicle binding assay; and all authors contributed to revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gary E. Gilbert, VA Boston Healthcare System, 1400 VFW Parkway, West Roxbury, MA 02132; e-mail: Gary_Gilbert@hms.harvard.edu or ggilbert@rics.bwh.harvard.edu.