Abstract
Two major pathways of human myeloid dendritic cell (DC) subset differentiation have previously been delineated. Langerhans cells (LCs) reside in epithelia in the steady state, whereas monocytes can provide dendritic cells (DCs) on demand in response to inflammatory signals. Both DC subset pathways arise from shared CD14+ monocyte precursors, which in turn develop from myeloid committed progenitor cells. However, the underlying hematopoietic mechanisms still remain poorly defined. Here, we demonstrate that the vitamin D3 receptor (VDR) is induced by transforming growth factor β1 during LC lineage commitment and exerts a positive role during LC generation. In contrast, VDR is repressed during interleukin-4 (IL-4)–dependent monocyte-derived DC (moDC) differentiation. We identified GATA-1 as a repressor of VDR. GATA-1 is induced by IL-4 in moDCs. Forced inducible expression of GATA-1 mimics IL-4 in redirecting moDC differentiation and vice versa, GATA-1 knockdown arrests moDC differentiation at the monocyte stage. Moreover, ectopic GATA-1 expression stabilizes the moDC phenotype under monocyte-promoting conditions in the presence of vitamin D3 (VD3). In summary, human myeloid DC subset differentiation is inversely regulated by GATA-1 and VDR. GATA-1 mediates the repression of VDR and enables IL-4–dependent moDC differentiation. Conversely, VDR is induced downstream of transforming growth factor β1 and is functionally involved in promoting LC differentiation.
Introduction
Two in vitro systems for the generation of human dendritic cells (DCs) are widely used for basic and clinically oriented research: monocyte-derived DCs (moDCs) and hematopoietic progenitor cell (HPC)–derived DCs. In the first system, granulocyte-macrophage colony-stimulating factor (GM-CSF) plus interleukin-4 (IL-4) are added to peripheral blood monocytes to generate moDCs.1 This system is most widely applied for generating human DCs. In the second model, CD34+ HPCs are stimulated in transforming growth factor β1 (TGF-β1)–supplemented serum-free cultures (containing basic DC cytokines GM-CSF, tumor necrosis factor α [TNFα], stem cell factor [SCF] with or without Flt3L) for generating Langerhans cells (LCs), a DC subtype that resides within epithelial tissue.2,3 Exclusion of TGF-β1 from this latter culture system abrogates LC differentiation and de-represses a program of monocyte generation from CD34+ cells.3 When these latter HPC cultures are initiated in the presence of serum without TGF-β1, 2 pathways of CD1a+ DCs are simultaneously generated, ie LCs and non-LC interstitial/dermal-type DCs.4 These populations arise from 2 separate precursor pathways at culture day 5. A CD1a+CD14− precursor gives rise to LCs, whereas a CD1a−CD14+ precursor can give rise to non-LC DCs, which share many features with moDCs generated from peripheral blood monocytes.4,5 This CD1a−CD14+ intermediate population can be further subdivided into 2 functional subsets based on CD11b (CD11b+CD14+ versus CD11b−CD14+ monocytic cells). CD11b+CD14+ cells can be induced by GM-CSF plus IL-4 to develop into intDC/moDCs or by M-CSF to macrophage differentiation. Conversely, CD11b−CD14+ intermediates, which represent early monocytic cells, retain LC differentiation capacity in the presence of TGF-β1.6 Therefore, a CD14+ monocytic precursor can be induced to develop along 2 separate pathways in vitro depending on IL-4 (moDCs) versus TGF-β1 (LCs) cytokine signals.
IL-4 versus TGF-β1 antagonize each other's function to induce moDCs versus LCs. Neutralizing anti–TGF-β1 mAb represses LC generation in favor of CD14+ monocyte generation.7 Similarly, the addition of IL-4 to TGF-β1–supplemented LC generation cultures of CD34+ cells represses LC differentiation in favor of inducing non-LC DC differentiation.7 Conversely, the addition of TGF-β1 to GM-CSF plus IL-4–containing moDC cultures of peripheral blood monocytes polarizes these cells toward an LC-like phenotype.8 In line with this, TGF-β1 in the epidermal microenvironment is critical for LC differentiation in vivo.9-11 Functional differences between moDCs versus LCs are increasingly recognized. However, very little is known about the molecular mechanisms controlling the lineage commitment of these cells to develop into different DC subsets (ie, LCs and non-LC moDCs).
We recently performed mechanistic studies on the involvement of family members of the nuclear hormone receptor system, ie, retinoid X receptor-alpha (RXRα) and vitamin D3 receptor (VDR) in myelopoiesis.12 RXRα and VDR form heterodimeric complexes that, on ligand binding (ligand: calcitriol, 1,25-vitamin D3; VD3), can induce monocyte differentiation of myeloid progenitors.13 By using a functional screen we recently identified the hematopoietic master transcription factor GATA-1 as a dominant interfering molecule of VDR:RXRα-induced monocyte differentiation from U937 model cells (S.T. and H.S., unpublished data, February 2006). Because monocyte genes are consistently repressed concomitant with LC2,3 or moDC1 subset differentiation, we studied whether the GATA-1/VDR:RXRα interdependency observed in our cell line model could possibly play a role in the development of monocytes to their resultant DC subsets. Moreover, a recent study has already implicated GATA-1 in murine DC development.14 We found that GATA-1 and VDR are inversely present in moDCs versus LCs and that moDCs require GATA-1 downstream of IL-4 for development. Furthermore, GATA-1 represses VDR in DC precursors. In sharp contrast, LC precursors up-regulate VDR in response to the linage-instructive signal TGF-β1, and VDR plays a positive role in LC differentiation.
Methods
Cytokines and reagents
The following cytokines were used: SCF, thrombopoietin, IL-4, TNFα, IL-15, IFN-α (PeproTech); TGF-β1 (R&D Systems); Flt3L (Amgen); GM-CSF (Novartis); 1α,25-dihydroxyvitamin D3 (1,25-VD3), 25-hydroxyvitamin D3 (25-VD3), doxycycline (DOX; Sigma-Aldrich); and VDR antagonist ZK159222 (Bayer Schering Pharma AG).
Cell isolation
Cord blood samples were collected during healthy full-term deliveries. Approval was obtained from the Medical University of Vienna Institutional Review Board for these studies. Informed consent was provided in accordance with the Declaration of Helsinki. CD34+ cells were isolated as previously described.15 CD14+ monocytes were isolated by magnetic-activated cell sorting (Miltenyi Biotec) from cord blood according to the manufacturer's instructions. For experiments shown in Figure 2B, CD34+ cells were sorted by fluorescence-activated cell sorting (FACS; FACSAria; BD Biosciences) into greater than 95% pure CD34+CD45RA+CD19− cells enriched in myeloid progenitors (FITC: CD34, clone 581; BD Biosciences; PE: CD45RA, clone MEM-56; Caltag Laboratories; APC: CD19, clone FJ25-C1; Caltag).
DC generation cultures
X-VIVO 15 medium (BioWhittaker) was used for primary cell cultures supplemented with GlutaMAX (2.5 mM; Invitrogen), penicillin/streptomycin (P/S; 125 U/mL each; Sigma). In addition, 10% FBS (Invitrogen) were added for moDC generation. CD34+ progenitor cells were expanded in Flt3L, SCF, and thrombopoietin (each 50 ng/mL) for 1 to 3 days. LCs were generated in GM-CSF (100 ng/mL), SCF (20 ng/mL), Flt3-L (50 ng/mL), TNFα (2.5 ng/mL), and TGF-β1 (0.5 ng/mL). MoDCs from expanded CD34+ cells were obtained in GM-CSF (100 ng/mL), SCF (20 ng/mL), Flt3-L (50 ng/mL), TNFα (2.5 ng/mL) for 4 days, followed by GM-CSF (100 ng/mL), IL-4 (25 ng/mL) for 3 days. Monocytes were cultured with GM-CSF (50 ng/mL) and IL-4 (25 ng/mL) for 6 days. Absolute cell numbers for LCs and moDCs generated from 2 × 104 CD34+ cells were in the range of 1 to 2 × 106 and 2 to 4 × 106, respectively. Monocyte cultures were initiated with 106 cells, and cell numbers stayed equivalent or only slightly dropped until day 6. Cell viability usually exceeded 90%, as assessed routinely by morphologic inspection and trypan blue dye exclusion. Cell cultures containing less than 90% viable cells were excluded from further analyses.
Cell lines
Phoenix (Ph) cells were kindly provided by G. P. Nolan (Stanford University). 293T cells were purchased from ATCC, and both were maintained in DMEM (Sigma)/10% FBS/l-glutamine (Sigma) and P/S. U937Te cells are described elsewhere.15 K562 cells were obtained from ATCC. K562 and U937Te cells were cultured in RPMI with 10% FBS, l-glutamine, and P/S.
Human foreskin tissue preparation and immunofluorescence staining
Noninflamed human foreskin from routine circumcisions was obtained as discarded material after ethical committee approval of the Medical University of Vienna (code EK555/2005). Frozen sections of foreskin were fixed and incubated with mouse–αVDR mAb (D-6; Santa Cruz Biotechnology) followed by goat–αmouse F(ab′)2 Alexa Flour 488 (Invitrogen), mouse–αhuman CD207-PE (DCGM4; Beckman Coulter), and mouse–αhuman CD45-APC (J33; Beckman Coulter). Immunostained sections were analyzed by a confocal laser scanning microscope (LSM 510; Carl Zeiss GmbH).
1,25-VD3 and 25-VD3 detection with radioimmunoassay
Skin pieces were homogenized in cold PBS by an Ultra Turrax (Janke-Kunkel GmbH). After centrifugation at 15 000 g for 10 minutes at 4°C, protein was precipitated with acetonitril; 25-VD3 and 1,25-VD3 were analyzed with radioimmunoassay kits from DiaSorin according to the manufacturer.
Vector constructs and gene transduction
Murine stem cell virus–based retroviral vector MIG-R1 was obtained from G.P. Nolan (Stanford University). Human GATA-1 cDNA was amplified by polymerase chain reaction (PCR) by using the primers 5′-TCCCCAGAGGCTCCATGGAG-3′ and 5′-GGGTTGTCCAGAATTCTGGCTAC-3′ from a human fetal liver cDNA library (Stratagene) and cloned into the EcoRI site of the MIG-R1. PINCO-VDR-GFP was kindly provided by A. Seshire (University of Frankfurt). The retroviral tetracycline-inducible system (Tet-on system) was described elsewhere.16 Briefly, the first vector encodes the Tet-activator, pTA-mCD8α. The second vector encodes human GATA-1 cDNA, which was cloned into the EcoRI site of the self-inactivating retroviral vector pHR-IRES-NGFR and pHR-IRES-GFP (kindly provided by F. Rossi, University of British Columbia), respectively. The packaging cell lines Ph-E, Ph-Gag-Pol (Ph-GP), and 293T were transfected as previously described.12,17 Retroviral infections of target cells was done as previously described.15 The silencing construct targeting GATA-1 (5′-ACGGCCTCTATCACAAGATGAA-3′) in a hairpin structure (OligoID: V2HS_114063) was inserted into the XhoI-MLuI sites of pGIPZ lentiviral vector (Open Biosystems). The nonsilencing pGIPZ (22-mer sequence 5′-ATCTCGCTTGGGCGAGAGTAAG-3′) was obtained from the same company. The GIPZ vector backbone has a GFP marker molecule to track infected cells. Lentiviral infections were carried out according to standard procedures18 for silencing experiments. In brief, 293T cells were cotransfected with pMD2.G and psPAX2 (Addgene Inc) and the respective GIPZ-shRNA silencing construct by using 1 μg/mL Polybrene (Sigma) to enhance lentiviral infections. Viral supernatants were used to infect monocytes, which were then differentiated to moDCs.
Flow cytometry
Western blot analysis
Western blot analysis was performed as previously described.17 Detection was carried out with goat–αGATA-1 (C-20), rabbit–αPU.1 (T-21), rabbit–αVDR (H81) (Santa Cruz), rabbit–αActin (A-2066; Sigma), sheep–α1α(OH)ase (Binding Site), followed by horseradish peroxide–conjugated donkey–αgoat or goat–αrabbit IgG (H+L) (Pierce) or rabbit-αsheep (Dako) antibodies. Cells (105) per lane were analyzed.
PCR
For reverse transcription (RT)–PCR, total RNA was isolated by using the RNeasy micro kit (QIAGEN) according to the manufacturer's instructions. DNA-free total RNA was subjected to cDNA synthesis by using oligo-dT primers and M-MuLV reverse transcriptase (Fermentas) according to the manufacturer's instructions. Quantitative real-time RT-PCR was performed as described.19 The following primers were used: VDR, 5′-CCAGTTCGTGTGAATGATGG-3′ and 5′-AGATTGGAGAAGCTGGACGA-3′; HPRT, 5′-GACCAGTCAACAGGGGACAT-3′ and 5′-AACACTTCGTGGGGTCCTTTTC-3′; GAPDH, 5′-GAAATCCCATCACCATCTTCCAGG-3′ and 5′-CGCGGCCATCACGCCACAGTTTCC-3′.
Statistical analysis
Data are presented as mean plus or minus SEM. Statistical analysis was performed using the paired, 2-tailed Student t test. P values less than .05 were considered significant.
Results
Transcription factors GATA-1 and VDR are inversely expressed in human myeloid DC subsets
To study mechanisms of human myeloid DC differentiation, we established 2 in vitro differentiation culture models, initiated (1) by CD34+ umbilical cord blood cells or (2) by peripheral blood monocytes (Figure 1A). The first model allows for generation of either TGF-β1–dependent LCs3 or, by a 2-step culture system, IL-4–dependent moDCs.5 These IL-4–dependent moDCs share many phenotypic properties with moDCs generated from peripheral blood monocytes.1,5 CD11b has been previously identified as the marker molecule that best discriminates IL-4–dependent moDCs cells from LCs.5 Therefore, we used CD11b as a marker for moDCs (Figure 1A). A phenotypic comparison of in vitro–generated LCs and moDCs is shown in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). LCs and moDC subsets were identified as CD207+CD1a+ and CD11b+CD1a+ cells, respectively (see gate settings in supplemental Figure 1A) and analyzed for informative marker molecules. LCs but not moDCs express CD207 and E-cadherin. Conversely, LCs lack CD11b (supplemental Figure 1A). Furthermore, LCs and moDCs lack CD14. Moreover, we confirmed previous observations5 that moDCs derived from CD34+ cells but not LCs express CD209 (DC-SIGN) and that moDCs express higher levels of CD1c than do LCs (supplemental Figure 1B). Therefore, the here described in vitro generated DC subsets phenotypically resemble DC populations extensively characterized in previous studies.5,20 We recently identified the transcription factor GATA-1 as a repressor of VD3-induced monocytopoiesis in U937 monocytic cells (S.T. and H.S., unpublished data, February 2006). Interestingly, ectopic GATA-1 expression decreases VDR mRNA expression levels compared with control transduced cells as shown from quantitative real-time RT-PCR analyses (Figure 1B). In line with this, a previous study has shown that monocytes down-regulate VDR expression during GM-CSF/IL-4–dependent moDC differentiation.21 These observations, together with a recent study that implicated GATA-1 in murine DC development,14 prompted us to perform a comparative analysis of GATA-1 and VDR in LCs versus moDCs. Western blot analyses showed that GATA-1 is detectable in GM-CSF/IL-4–dependent moDCs generated from CD34+ cells or peripheral blood monocytes, whereas LCs lack GATA-1 (Figure 1C). Conversely, VDR is expressed at higher levels in LCs compared with moDCs (Figure 1C). Therefore, GATA-1 and VDR are inversely expressed in these DC subsets. Time kinetics Western blot analyses of GM-CSF/IL-4–dependent moDC cultures has shown that GATA-1 expression is absent in monocytes at culture initiation (day 0). However, GATA-1 expression appears at day 2 and gradually increases thereafter until day 6 (Figure 1D). Conversely, VDR is expressed in monocytes (day 0) and is gradually repressed by day 2 during GM-CSF/IL-4–dependent moDC differentiation (Figure 1D top panel). Phenotypic analyses have shown that at day 3, the monocyte marker CD14 was scarcely detectable and that approximately half of the population expressed CD1a, whereas at day 6, more than 90% of cells were CD1a+CD11b+CD14− (Figure 1D bottom panel). These results indicate that GATA-1 up-regulation correlates with the differentiation of CD1a+CD11b+ moDCs between day 3 and day 6, as well as with the gradual repression of VDR during GM-CSF/IL-4–dependent moDC differentiation. In summary, GATA-1 and VDR are inversely expressed in DC subsets, suggesting a differential role during myeloid DC subset differentiation.
GATA-1 represses VDR and is inversely expressed by myeloid DC subsets. (A) Schematic representation of culture models for LC and moDC differentiation used in this study. (B) Quantitative real-time RT-PCR analyses of VDR mRNA expression in GATA-1–transduced monocytic cells. U937Te cells were transduced with a retroviral vector encoding GATA-1-IRES-GFP or empty control vector (CTRL). GFP+ cells were sorted by FACS 48 hours after infection. mRNA was extracted from 105 cells. Cells are analyzed for VDR mRNA levels relative to HPRT mRNA (n = 3; *P < .05). (C) Representative Western blot analysis of VDR and GATA-1 protein levels in DC subsets (n = 3). CD34+-derived moDCs and LCs were generated from CD34+ cord blood cells; moDCs were generated from blood monocytes. (D) Fresh blood monocytes or monocytes during culture (days 2, 3, and 6) in the presence of GM-CSF/IL-4 (moDC cultures) were analyzed by Western blot (top) or by FACS (bottom; day 0, day 3, day 6). Each number in a quadrant represents the percentage of cells in the quadrant. Data are representative of 4 independent donors.
GATA-1 represses VDR and is inversely expressed by myeloid DC subsets. (A) Schematic representation of culture models for LC and moDC differentiation used in this study. (B) Quantitative real-time RT-PCR analyses of VDR mRNA expression in GATA-1–transduced monocytic cells. U937Te cells were transduced with a retroviral vector encoding GATA-1-IRES-GFP or empty control vector (CTRL). GFP+ cells were sorted by FACS 48 hours after infection. mRNA was extracted from 105 cells. Cells are analyzed for VDR mRNA levels relative to HPRT mRNA (n = 3; *P < .05). (C) Representative Western blot analysis of VDR and GATA-1 protein levels in DC subsets (n = 3). CD34+-derived moDCs and LCs were generated from CD34+ cord blood cells; moDCs were generated from blood monocytes. (D) Fresh blood monocytes or monocytes during culture (days 2, 3, and 6) in the presence of GM-CSF/IL-4 (moDC cultures) were analyzed by Western blot (top) or by FACS (bottom; day 0, day 3, day 6). Each number in a quadrant represents the percentage of cells in the quadrant. Data are representative of 4 independent donors.
Functional involvement of VDR in TGF-β1–dependent LC differentiation
Next, we focused on the role of VDR in LCs. Analysis of human skin samples confirmed that CD45+CD207+ LCs express VDR in vivo. It can be seen from the high-power magnification insert in Figure 2A that LCs show a nuclear VDR expression pattern resembling that observed in keratinocytes, thus confirming and extending previous observations.22 Because LCs require TGF-β1 for their generation from progenitors,2 we next asked whether TGF-β1 induces VDR concomitant with LC lineage differentiation. Thus, myeloid progenitors (CD34+CD45RA+CD19− cells) were isolated and stimulated with LC-promoting cytokines in the absence or presence of TGF-β1 for 7 days. The addition of TGF-β1 to these cultures induces CD1a+CD207+ LC differentiation, whereas, in the absence of TGF-β1, cells differentiate into CD11b+CD14+ monocytes (Figure 2B). Quantitative real-time RT-PCR analysis showed strong induction of VDR within 6 hours in response to TGF-β1 stimulation of myeloid progenitors (Figure 2B bar diagram). Because we observed VDR induction in response to the LC lineage instructive signal TGF-β1, we then addressed whether ectopic VDR might promote LC differentiation. Thus, CD34+ cells were transduced with a retroviral vector encoding VDR-IRES-GFP or an empty control vector. Subsequently, cells were induced to differentiate into LCs in the presence of TGF-β1. For comparison, TGF-β1 was omitted from parallel cultures. For phenotypic analysis of gene-transduced cells, GFP+ cells were gated in a separate FACS diagram (data not shown). In the presence of TGF-β1, VDR-transduced cells showed strongly increased percentages of CD1a+CD207+ LCs relative to empty vector control (Figure 2C FACS and bar diagrams), indicating that VDR promotes LC differentiation. This effect was abrogated in the absence of TGF-β1 (Figure 2C FACS and bar diagram), showing that VDR expression alone is not sufficient to induce LC differentiation. We confirmed that VDR-transduced LCs lack marker molecules associated with moDC differentiation or monocytes (CD11b− and CD14−; supplemental Figure 1C). Because VDR is repressed during GM-CSF/IL-4–dependent moDC generation, we next determined the role of ectopic VDR during this differentiation process. Therefore, gen-transduced progenitor cells were induced to develop into GM-CSF/IL-4–dependent CD1a+CD11b+ moDCs according to Figure 1A. Ectopic VDR expression reduced the generation of GM-CSF/IL-4–dependent CD1a+CD11b+ moDCs (Figure 2D), indicating an inhibitory effect of VDR expression on moDC differentiation. To address whether endogenous VDR is required for LC lineage differentiation, we generated LCs in the presence or absence of the VDR antagonist ZK159222.23,24 The addition of ZK159222 to LC differentiation cultures inhibited the generation of CD1a+CD207+ LCs (Figure 2E). Taken together, these experiments indicate that VDR induction is functionally involved in LC differentiation downstream of TGF-β1.
VDR promotes TGF-β1–dependent LC differentiation. (A) Human infant foreskin assessed by immunofluorescence staining of cryosections for the expression of CD45 (blue), CD207 (red), and VDR (green). Immunostained sections were analyzed on a Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss GmbH) using immersion oil as imaging media and a 40×1.3 NA oil immersion objective (Plan-Neofluar; Zeiss). FITC, PE, APC channels were acquired by sequential scanning using 488-, 543-, or 633-nm laser lines for excitation; HFT 488, HFT 488/543, or HFT 514/633 beam-splitters for separation; and BP 505-530 nm, BP 560-615 nm, or LP 650 nm and Photomultiplier (PMT) for detection of the signals. Scale bar, 50 μm. The inset shows an enlarged view of part of the figure. (B) CD34+CD45RA+CD19− umbilical cord blood cells were sorted by FACS and cultivated with the indicated cytokines. Cell numbers of sorted fractions ranged from 7.25 × 104 to 2.3 × 105 (mean, 1.3 × 105; n = 6). Cultures were initiated with 1.2 × 104 to 4.1 × 104 cells (mean, 2.3 × 104; n = 6). FACS diagrams represent day 7–generated cells analyzed for informative marker molecules (± TGF-β1). In the bar diagram, VDR mRNA levels were determined by quantitative real-time RT-PCR at 0, 6 and 24 hours. Values are normalized to HPRT (n = 4). (C) CD34+ cells were transduced with a retroviral vector encoding VDR-IRES-GFP or empty control vector (CTRL) and were then cultured in LC conditions (GM-CSF, SCF, FL, TNFα) in the presence (top panel) or absence (bottom panel) of TGF-β1. Day 7–generated GFP+ cells were analyzed for CD1a versus CD207. Bars represent the mean of percentages (± SEM) CD1a+CD207+ cells observed in 4 independent experiments. (D) CD34+ cells were transduced with a retroviral vector encoding VDR-IRES-GFP or empty control vector (CTRL) and were then cultured in moDC conditions (see Figure 1A). Day 7–generated GFP+ cells were analyzed for CD11b versus CD207. Data are representative of 3 independent experiments. (B-D) Each number in a quadrant represents the percentage of cells in the quadrant. (E) LCs were generated from CD34+ cells in the absence or presence of the VDR antagonist ZK159222 (10−6 M). At day 7 cells were harvested and analyzed for CD207 versus CD1a. Data are representative of 3 independent experiments.
VDR promotes TGF-β1–dependent LC differentiation. (A) Human infant foreskin assessed by immunofluorescence staining of cryosections for the expression of CD45 (blue), CD207 (red), and VDR (green). Immunostained sections were analyzed on a Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss GmbH) using immersion oil as imaging media and a 40×1.3 NA oil immersion objective (Plan-Neofluar; Zeiss). FITC, PE, APC channels were acquired by sequential scanning using 488-, 543-, or 633-nm laser lines for excitation; HFT 488, HFT 488/543, or HFT 514/633 beam-splitters for separation; and BP 505-530 nm, BP 560-615 nm, or LP 650 nm and Photomultiplier (PMT) for detection of the signals. Scale bar, 50 μm. The inset shows an enlarged view of part of the figure. (B) CD34+CD45RA+CD19− umbilical cord blood cells were sorted by FACS and cultivated with the indicated cytokines. Cell numbers of sorted fractions ranged from 7.25 × 104 to 2.3 × 105 (mean, 1.3 × 105; n = 6). Cultures were initiated with 1.2 × 104 to 4.1 × 104 cells (mean, 2.3 × 104; n = 6). FACS diagrams represent day 7–generated cells analyzed for informative marker molecules (± TGF-β1). In the bar diagram, VDR mRNA levels were determined by quantitative real-time RT-PCR at 0, 6 and 24 hours. Values are normalized to HPRT (n = 4). (C) CD34+ cells were transduced with a retroviral vector encoding VDR-IRES-GFP or empty control vector (CTRL) and were then cultured in LC conditions (GM-CSF, SCF, FL, TNFα) in the presence (top panel) or absence (bottom panel) of TGF-β1. Day 7–generated GFP+ cells were analyzed for CD1a versus CD207. Bars represent the mean of percentages (± SEM) CD1a+CD207+ cells observed in 4 independent experiments. (D) CD34+ cells were transduced with a retroviral vector encoding VDR-IRES-GFP or empty control vector (CTRL) and were then cultured in moDC conditions (see Figure 1A). Day 7–generated GFP+ cells were analyzed for CD11b versus CD207. Data are representative of 3 independent experiments. (B-D) Each number in a quadrant represents the percentage of cells in the quadrant. (E) LCs were generated from CD34+ cells in the absence or presence of the VDR antagonist ZK159222 (10−6 M). At day 7 cells were harvested and analyzed for CD207 versus CD1a. Data are representative of 3 independent experiments.
Endogenous GATA-1 mediates moDC differentiation downstream of IL-4
Because GATA-1 was specifically expressed in moDCs (Figure 1C), we next addressed the functional role of endogenous GATA-1 in these cells. MoDCs generated in response to GM-CSF plus IL-4 express GATA-1, whereas fresh monocytes lack detectable GATA-1 expression (Figure 3A). GATA-1 expression levels in moDCs are lower than in K562 leukemia cells, which typically serve as a reference cell line, given its abundant GATA-1 expression (Figure 3A). Importantly, omission of IL-4 from moDC generation cultures markedly reduces GATA-1 expression levels (Figure 3A), suggesting that IL-4 induces GATA-1 expression. This interpretation is supported by the time-kinetic experiment shown in Figure 1D, showing that GATA-1 protein levels gradually increase during IL-4–induced moDC differentiation from day 2 to day 6 and that GATA-1 expression positively correlates with the induction of a CD1a+CD11b+ moDC phenotype. We then asked whether endogenous GATA-1 is required for moDC differentiation downstream of IL-4. Therefore, we performed loss-of-function experiments of GATA-1 in moDC differentiation by using a lentiviral GATA-1 shRNA vector. Control experiments confirmed that this silencing vector reduces GATA-1 protein levels in K562 cells (Figure 3B top panel) and in moDCs (Figure 3B bottom panel) relative to nonsilencing shRNA control vector. Monocytes were transduced with shGATA-1 or a nonsilencing control and were differentiated to moDCs in the presence of GM-CSF plus IL-4 for 7 days. FACS analysis of GFP+ cells (vector encodes GFP; see simplified vector graph in Figure 3C) showed that shGATA-–expressing cells show a strong decrease in the percentages of CD1a+CD11b+ moDCs compared with control transduced cells (62% for control; 5% for shGATA-1; Figure 3C lower FACS diagrams). In addition, most shGATA1-transduced cells remained CD14+. These results show that silencing of endogenous GATA-1 impairs moDC differentiation. As a control experiment, we determined the effect of silencing GATA-1 during LC differentiation. Given our observations that LCs lack GATA-1 (Figure 1C), we expected that silencing of GATA-1 would fail to impair LC differentiation. Confirming this assumption, CD34+ progenitor cells transduced with shGATA-1 were equally effective as control transduced cells in differentiating into LCs (data not shown). Together these data indicate that GATA-1 is induced downstream of IL-4 during moDC differentiation and that GATA-1 is functionally required for moDC differentiation.
Essential role of GATA-1 downstream of IL-4 during moDC differentiation. (A) Peripheral blood monocytes were stimulated with GM-CSF plus IL-4 or GM-CSF alone for 6 days. Fresh monocytes or day 6–generated cells were analyzed for GATA-1 expression by using Western blot analysis. K562 cells are compared. Data are representative of 4 independent donors. (B) Silencing of GATA-1. Western blot of GATA-1 and actin expression of not infected, nonsilencing or GATA-1 shRNA infected K562 cells (top) and moDCs (bottom) at day 7 of differentiation. GFP+ cells were purified before analysis. (C) Monocytes were transduced with a lentiviral vector encoding nonsilencing or GATA-1 shRNA sequences (ie, shGATA-1-GFP or control). A simplified graphical representation of the vector backbone is shown. Infected monocytes were induced by GM-CSF/IL-4 to differentiate to moDCs. Gated GFP+ cells were analyzed by FACS for the indicated markers. FACS blots are representative of 2 independent experiments. Cell cultures were initiated with 106 monocytes, and cell recovery at day 7 was greater than 70%.
Essential role of GATA-1 downstream of IL-4 during moDC differentiation. (A) Peripheral blood monocytes were stimulated with GM-CSF plus IL-4 or GM-CSF alone for 6 days. Fresh monocytes or day 6–generated cells were analyzed for GATA-1 expression by using Western blot analysis. K562 cells are compared. Data are representative of 4 independent donors. (B) Silencing of GATA-1. Western blot of GATA-1 and actin expression of not infected, nonsilencing or GATA-1 shRNA infected K562 cells (top) and moDCs (bottom) at day 7 of differentiation. GFP+ cells were purified before analysis. (C) Monocytes were transduced with a lentiviral vector encoding nonsilencing or GATA-1 shRNA sequences (ie, shGATA-1-GFP or control). A simplified graphical representation of the vector backbone is shown. Infected monocytes were induced by GM-CSF/IL-4 to differentiate to moDCs. Gated GFP+ cells were analyzed by FACS for the indicated markers. FACS blots are representative of 2 independent experiments. Cell cultures were initiated with 106 monocytes, and cell recovery at day 7 was greater than 70%.
Ectopic GATA-1 expression during LC differentiation can induce CD11b+CD1a+ cells that phenotypically resemble moDCs
We studied whether ectopic GATA-1 or IL-4 might show similar effects on LC differentiation. It was previously shown that TGF-β1 induces LCs, whereas IL-4 addition to pregenerated day 5 LC precursors dominates over TGF-β1 and shifts the overall differentiation pattern toward a CD11b+ moDC phenotype7 (Figure 4A). We applied a Tet-on inducible system16 for specifically expressing GATA-1 in day 5–generated LC precursors. Hence, CD34+ cells expressing DOX-inducible GATA-1-IRES-NGFR or empty control were stimulated in LC-promoting cytokines for 5 days in the absence of DOX (Figure 4B left). Thereafter, DOX was added from day 5 to day 7 (Figure 4B). At day 7, NGFR+ cells were gated and analyzed for their phenotype (Figure 4B right). The here used Tet-on system allowed tightly regulated DOX-dependent induction of gene expression as visualized by NGFR, because in the absence of DOX cells remained NGFR− (Figure 4C). DOX-induced GATA-1 expression promoted the generation of CD11b+CD1a+ cells (FACS diagrams in Figure 4B and bar statistics in Figure 4D). Control experiments confirmed that GATA-1 expression in GM-CSF/IL-4–induced moDCs fails to inhibit CD1a expression (supplemental Figure 2). This observation is in line with above-described induction of endogenous GATA-1 during moDC differentiation (Figures 1D and 3A). Given the above-mentioned observations that ectopic GATA-1 inhibits VDR in U937 cells (Figure 1B), we then studied whether GATA-1 similarly inhibits VDR expression in LC precursors. Thus, we transduced cells under LC generation conditions with GATA-1-IRES-GFP or empty vector control, followed by cell sorting of GFP+ cells 48 hours after transduction. In line with our data in U937 cells (Figure 1B), ectopic GATA-1 clearly reduces VDR mRNA expression in LC precursors, as shown from quantitative real-time RT-PCR analysis (Figure 4E). Together these experiments show that ectopic GATA-1 mimics IL-4 stimulation in inducing CD1a+CD11b+ moDCs from day 5–generated LC precursors. Furthermore, ectopic GATA-1 in LC precursors diminishes VDR mRNA expression.
Constitutive and inducible GATA-1 expression in LC precursors. (A) IL-4 promotes CD11b+CD1a+ cells at the expense of CD207+CD1a+cells when added at day 5 to LC generation cultures. CD34+ cells were induced to differentiate to LCs. Day 5–generated cells were subcultured in LC cytokines in the absence or presence of IL-4 (25 ng/mL) for 4 days. At day 9 cells were harvested and analyzed for CD207 or CD11b versus CD1a. Data are representative of 3 independent experiments. (B-D) Tet-on-inducible system in primary LC precursors. CD34+ cells were transduced with retroviral vectors encoding Tet-activator-IRES-lyt2, DOX-inducible GATA-1-IRES-NGFR, or empty vector control. Cells were stimulated with LC-promoting cytokines for 5 days. DOX (2 μg/mL) was added from day 5 to day 7. (B) FACS diagrams show day 5–generated cells (left) or day 7–generated NGFR+ cells (right) analyzed for CD207 or CD11b versus CD1a. (C). FACS diagrams represent cells at day 7 analyzed for NGFR expression. (A-C) Each number in a quadrant represents the percentage of cells in the quadrant. (D) Bars represent mean percentages (± SEM; n = 3) of CD1a+CD11b+ cells from control vector or GATA-1–transduced cells at day 5 (total culture) and day 7 (gated NGFR+ cells); *P < .05. (E) LC generation cultures were transduced at day 3 to day 4 with a retroviral vector encoding VDR-IRES-GFP or empty control vector. Forty-eight hours after transduction, GFP+ cells were isolated by using FACS. Equal numbers of GFP+ cells were used for quantitative real-time RT-PCR analysis. Bars represent VDR mRNA levels in 1 representative experiment (cell numbers in PCR experiments: 3 × 104 to 2 × 105; n = 4).
Constitutive and inducible GATA-1 expression in LC precursors. (A) IL-4 promotes CD11b+CD1a+ cells at the expense of CD207+CD1a+cells when added at day 5 to LC generation cultures. CD34+ cells were induced to differentiate to LCs. Day 5–generated cells were subcultured in LC cytokines in the absence or presence of IL-4 (25 ng/mL) for 4 days. At day 9 cells were harvested and analyzed for CD207 or CD11b versus CD1a. Data are representative of 3 independent experiments. (B-D) Tet-on-inducible system in primary LC precursors. CD34+ cells were transduced with retroviral vectors encoding Tet-activator-IRES-lyt2, DOX-inducible GATA-1-IRES-NGFR, or empty vector control. Cells were stimulated with LC-promoting cytokines for 5 days. DOX (2 μg/mL) was added from day 5 to day 7. (B) FACS diagrams show day 5–generated cells (left) or day 7–generated NGFR+ cells (right) analyzed for CD207 or CD11b versus CD1a. (C). FACS diagrams represent cells at day 7 analyzed for NGFR expression. (A-C) Each number in a quadrant represents the percentage of cells in the quadrant. (D) Bars represent mean percentages (± SEM; n = 3) of CD1a+CD11b+ cells from control vector or GATA-1–transduced cells at day 5 (total culture) and day 7 (gated NGFR+ cells); *P < .05. (E) LC generation cultures were transduced at day 3 to day 4 with a retroviral vector encoding VDR-IRES-GFP or empty control vector. Forty-eight hours after transduction, GFP+ cells were isolated by using FACS. Equal numbers of GFP+ cells were used for quantitative real-time RT-PCR analysis. Bars represent VDR mRNA levels in 1 representative experiment (cell numbers in PCR experiments: 3 × 104 to 2 × 105; n = 4).
Ectopic GATA-1 allows CD1a+CD11b+ moDC differentiation during VD3-induced monopoiesis
The addition of 1.25-VD3 to DC generation cultures is well described to inhibit CD1a+ DCs in favor of monocyte generation.25-28 Consequently, GATA-1–mediated down-regulation of VDR may possibly inhibit the effects of the VDR ligand 1.25-VD3 on DC differentiation. Therefore, we hypothesized that low levels of ectopic GATA-1 expression as observed in moDCs (Figures 1D and 3A) may desensitize precursor cells to VD3-mediated inhibition of DC generation. Moreover, given the above-described ectopic expression experiments (Figure 4B), GATA-1 expression could possibly induce a CD1a+CD11b+ moDC phenotype. Consequently, increased percentages of CD1a+CD11b+ moDCs may occur among GATA-1low–expressing cells in the presence of 1.25-VD3. In contrast, high GATA-1 expression levels typically found in erythroid cells might generally inhibit monocyte and moDC differentiation. To test these assumptions, we transduced CD34+ progenitors with GATA-1-IRES-GFP and cultured them in LC differentiation conditions in the presence of 1.25-VD3. As expected, GFPlo or GFPhi fractions sorted by FACS differed in GATA-1 protein expression intensity (Figure 5A Western blot). FACS analysis showed that gated GFPlo (GATA-1lo) cells indeed included a significantly elevated percentage of CD1a+CD11b+ cells compared with empty vector control or GFPhi cells (Figure 5A FACS and bar diagrams). Conversely, the generation of CD1a+CD11b+ cells was abrogated when gating on GFPhi (GATA-1hi) cells (Figure 5A FACS diagrams). Similarly, in congruence with the above-mentioned assumption, high or low GATA-1 levels inhibited monocyte generation (data not shown). These data indicate that low levels of GATA-1 restore CD1a+CD11b+ cell differentiation in the presence of 1.25-VD3. Because GATA-1 inhibits VDR expression and thereby may desensitize progenitors to VD3, we expected that inhibition of VDR:RXRα signaling could duplicate this effect. Therefore, we compared ectopic GATA-1 side by side with ectopic RXRαΔ (an N-terminally truncated version of the VDR heterodimerization partner RXRα), which we recently described to effectively inhibit VDR-dependent monopoiesis.12 It can be seen in Figure 5B that RXRαΔ induced the expression of CD1a in LC generation cultures in the presence of 1.25-VD3. Gating on GFPlo versus GFPhi cells indicated a dose-dependent effect of RXRαΔ on promoting the generation of CD1a+CD11b+ cells (Figure 5B). Parallel analyses showed that low GATA-1 (GFPlo) versus high RXRαΔ (GFPhi) expression results in equivalent increases in percentages of CD1a+CD11b+ DCs (compare bar diagrams in Figure 5A with 5B). Taken together, these experiments support that low levels of GATA-1 are sufficient to promote moDC differentiation under 1.25-VD3–induced monocyte-promoting conditions, indicating that GATA-1 desensitizes cells to 1.25-VD3 by the down-regulation of VDR expression. In subsequent experiments we tested whether ectopic (nonregulated) VDR levels in the presence of VDR agonist 1.25-VD3 might counteract GATA-1–mediated effects. U937 monocytic cells show repressed endogenous VDR in response to GATA-1 expression (Figure 1B), similarly as observed for primary cells (Figure 4E). We compared the effects of ectopic GATA-1 versus GATA-1 plus VDR on CD11b expression by U937 cells. As can be seen from supplemental Figure 3, ectopic VDR in the presence of VDR agonist 1.25-VD3 impaired CD11b expression (supplemental Figure 3).
GATA-1 induction mimics VDR-signal inhibition in reestablishing DC development in the presence of 1.25-VD3. CD34+ cells were transduced with GATA-1-IRES-GFP, RXRαΔ-IRES-GFP, or empty vector control. Forty-eight hours after transduction, cells were plated in cultures supplemented with LC-promoting cytokines in the presence of 62.5 nmol/L 1.25-VD3 for 7 days. GFPneg, GFPlo, and GFPhi cells were separately gated and were analyzed for CD1a and CD11b expression. Bar diagrams represent mean percentages (± SEM) of CD1a+ CD11b+ cells in gene-transduced populations as indicated. Each number in a quadrant represents the percentage of cells in the quadrant. (A) GATA-1 versus control vector, n = 5; (B) RXRαΔ versus control vector, n = 3. Western blot shows GATA-1 and actin expression of GFPlo versus GFPhi GATA-1-IRES-GFP–transduced cells sorted by FACS. Data are representative of 3 independent experiments.
GATA-1 induction mimics VDR-signal inhibition in reestablishing DC development in the presence of 1.25-VD3. CD34+ cells were transduced with GATA-1-IRES-GFP, RXRαΔ-IRES-GFP, or empty vector control. Forty-eight hours after transduction, cells were plated in cultures supplemented with LC-promoting cytokines in the presence of 62.5 nmol/L 1.25-VD3 for 7 days. GFPneg, GFPlo, and GFPhi cells were separately gated and were analyzed for CD1a and CD11b expression. Bar diagrams represent mean percentages (± SEM) of CD1a+ CD11b+ cells in gene-transduced populations as indicated. Each number in a quadrant represents the percentage of cells in the quadrant. (A) GATA-1 versus control vector, n = 5; (B) RXRαΔ versus control vector, n = 3. Western blot shows GATA-1 and actin expression of GFPlo versus GFPhi GATA-1-IRES-GFP–transduced cells sorted by FACS. Data are representative of 3 independent experiments.
Discussion
Shared CD14+ monocytic precursors can give rise to LCs in response to TGF-β1 stimulation or to interstitial/inflammatory-type DCs in response to GM-CSF plus IL-4 independently of TGF-β1.7 Here, we studied the molecular mechanisms underlying separate lineage differentiation of monocytic cells to LCs or moDCs.
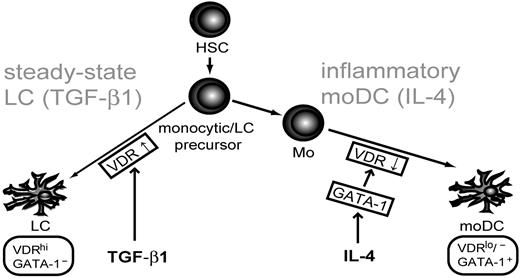
This study identified 2 key regulators of myeloid DC subset specification, VDR and GATA-1. We found that VDR is rapidly induced downstream of TGF-β1 during LC lineage specification. Conversely, GATA-1 is induced downstream of IL-4 during moDC differentiation. Gain- and loss-of-function analyses showed that VDR and GATA-1 are involved in LC and moDC differentiation. Moreover, we identified GATA-1 as a repressor of VDR in moDC precursors. Specifically, IL-4–induced moDC generation from blood monocytes is associated with diminished VDR expression. Similarly, ectopic GATA-1 represses VDR in moDC precursors. In this function, GATA-1 resembled a repressor of VDR signaling in desensitizing DC precursors to suppressive effects of 1.25-VD3. In line with these findings, ectopic VDR inhibited moDC differentiation. A schematic summary of these findings is presented in Figure 6.
Model for the role of GATA-1 and VDR in myeloid DC subset generation at steady state versus inflammatory conditions. VDR is induced downstream of TGF-β1 during LC differentiation. In contrast, DCs derived from monocytes in response to IL-4 down-regulate VDR. IL-4 induces GATA-1, which in turn represses VDR. Our study showed that VDR and GATA-1 are functionally involved in myeloid DC subset differentiation.
Model for the role of GATA-1 and VDR in myeloid DC subset generation at steady state versus inflammatory conditions. VDR is induced downstream of TGF-β1 during LC differentiation. In contrast, DCs derived from monocytes in response to IL-4 down-regulate VDR. IL-4 induces GATA-1, which in turn represses VDR. Our study showed that VDR and GATA-1 are functionally involved in myeloid DC subset differentiation.
GM-CSF/IL-4–induced moDCs phenotypically resemble inflammatory dendritic epidermal cells (IDECs) present in lesions of the atopic eczema/dermatitis syndrome or in certain other inflammatory skin diseases. IDECs lack LC characteristics, appear at inflammatory sites, and share with GM-CSF/IL-4–induced moDCs the expression of CD206, CD11b, and CD1a.29 Furthermore, IDECs and moDCs share many phenotypic characteristics with dermal/interstitial DCs found at steady state.29 Therefore, reciprocal GATA-1 and VDR expression by LCs versus moDCs might be critical regulators of myeloid DC fates in steady-state versus inflammatory conditions (Figure 6). It will be interesting to study to which extent the here-described GATA-1– and VDR-dependent regulation could apply to other candidate inflammatory DC subsets.30
Low GATA-1 levels in moDCs are reminiscent of low GATA-1 expression in previous studies of other leukocyte subsets, including mast cells, eosinophils31,32 and murine DC subsets.14 We showed that GATA-1 is induced in response to IL-4 stimulation during moDC differentiation. Importantly, shRNA knock-down experiments showed a functional requirement for endogenous GATA-1 in promoting moDC differentiation. Furthermore, ectopic GATA-1 diminished VDR mRNA expression, and low levels of GATA-1 were sufficient to desensitize DC precursors to inhibitory effects of 1,25-VD3. Repression of VDR by GATA-1 might desensitize DCs to possible inhibitory effects of VD3 metabolites and thereby may enable efficient antigen-presenting function. Taken together, GATA-1 represses VDR and is functionally involved in promoting moDC differentiation downstream of IL-4.
It was previously shown that DCs can produce IL-4 under certain conditions in an autocrine fashion.33,34 Moreover, exposure of DCs to IL-4 was shown to be necessary to induce IL-4 production in these cells.35 Most notably, GATA-1 binding sites were found in an IL-4 intronic enhancer element,36 and these sites were found to be essential for IL-4 expression in mast cells.37 Thus, GATA-1 in DCs might represent part of an IL-4–driven positive feedback loop. This possibility needs to be further elucidated.
We found that VDR mRNA is decreased within 48 hours after ectopic GATA-1 expression in primary cells and U937 cells; however, the underlying mechanism remains to be identified. One possibility is that GATA-1 acts as a direct transcriptional repressor of VDR, given the existence of several potential GATA-1 binding sites in the VDR promoter. Alternatively, but not mutually exclusive, GATA-1 might inhibit VDR mRNA expression by an indirect mechanism. Interestingly, the 5-kb VDR promoter region contains 3 potential PU.1 binding sites. Because GATA-1 binds and antagonizes PU.1,38-40 its negative effect on VDR expression might be secondarily mediated by PU.1 antagonism. Future studies should address this question.
We observed that overexpression of GATA-1 stabilizes the monocyte-like DC phenotype even in the presence of VDR agonist 1,25-VD3, therefore indicating that the actions of GATA-1 are dominant over those of vitamin D. Our data in U937 model cells seem to support that VDR down-regulation is critical for GATA-1–mediated induction of CD11b. Specifically, ectopic (nonregulated) VDR in the presence of VDR agonist partially impaired GATA-1–mediated induction of CD11b. Detailed mechanistic experiments in primary DCs are required to further study this point.
One key finding of our study was that VDR function intersects with TGF-β1 during LC lineage specification. Evidence for this is presented by the observations that purified myeloid progenitors rapidly up-regulate VDR in response to TGF-β1 signaling under LC lineage-instructive stimulation conditions and that ectopic VDR promotes TGF-β1–dependent LC differentiation. In the absence of TGF-β1, progenitors expressed low VDR levels and differentiated along a monocyte pathway. By Using identical cytokine stimulation conditions as used in this study, we previously provided evidence that TGF-β1 redirects monocytes to LC differentiation in single-cell cultures of CD34+ cells.3 In support of these data, we have also shown that the addition of a VDR antagonist inhibited LC differentiation.
VDR immunohistology analyses of human skin samples showed a general bright staining pattern in epidermis. In particular, CD207+ epidermal LCs in situ showed a nuclear VDR expression pattern reminiscent of keratinocytes. Interestingly, unligated VDR was previously shown to induce keratinocyte differentiation.41 In support of this, deficiency of 1α-hydroxylase [1α(OH)ase, Cyp27B1], an enzyme converting 25-VD3 to the biologically active metabolite 1,25-VD3, in mice has no skin phenotype,42 whereas VDR-deficient mice show strongly increased keratinocyte proliferation and epidermal thickening.43 Several pieces of evidence indicate that unligated VDR might similarly promote LC differentiation in the absence of its ligand 1,25-VD3. First, the use of a serum-free culture model devoid of any VD3 showed that VDR strongly promotes LC differentiation. Second, the addition of a VDR inhibitor interfered with LC differentiation in these serum-free cultures devoid of VD3. Third, we detected only low levels of 25-VD3 in human skin (data not shown). Specifically, whereas serum levels of 1,25-VD3 and its precursor 25-VD3 are known to range from 40 to 150 pmol/L and 75 to 150 nmol/L, respectively,44,45 skin samples contained only low levels of 25-VD3 (9.0 and 9.5 nmol/L in 2 independent skin specimens) and 1,25-VD3 remained undetectable. However, LCs expressed similar amounts of 1α-hydroxylase [1α(OH)ase, Cyp27B1] as did CD34+ cells and moDCs21,46 (data not shown). Therefore, LCs might be constitutively exposed to low amounts of bioactive 1,25-VD3. In this respect it is interesting that 1,25-VD3 produced by DCs can instruct T cells to express CCR10 and to migrate toward the epithelial chemokine CCL27.47 In an attempt to mimic LC exposure to VD3 metabolites, we found that low concentrations of 25-VD3 (6.25 nmol/L, ie, in the range detected in skin) failed to inhibit LC differentiation, whereas 10-fold higher amounts (serum concentrations) abrogated LC differentiation in favor of monocyte induction (data not shown). In conclusion our data support a model whereby VDR is induced downstream of the LC-linage instructive TGF-β1 signal and promotes LC differentiation from monocytic cells in the epidermis, a process that could be independent of its ligand 1.25-VD3.
The here-presented findings are based on retroviral vector–mediated gain- and loss-of-function analyses of human primary DC subsets. Additional studies are required to address how GATA-1 and VDR alter DC subset-associated functions.
In conclusion, TGF-β1, the key cytokine for LC differentiation, induces VDR under LC lineage instructive conditions. Given that TGF-β1–dependent LC differentiation is an epithelia-specific process, this pathway seems to be operative in LC renewal in epithelia. In contrast, IL-4 induces GATA-1 during moDC differentiation and may thereby enable this DC subset to differentiate within inflammatory sites. We identified GATA-1 as a repressor of VDR in moDCs. Repression of VDR in DCs may desensitize them to VD3-mediated inhibition and hence allow a stable DC phenotype. Therefore, low VDR in these DCs may very well be critical for the induction of effective antigen-specific immunity to pathogens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank W. Ellmeier, J. Stoeckl, M. Epstein, and H. Ramsey for critically reading the manuscript and for helpful discussions.
This work was supported by the Austrian Science Fund (grants START-Y156, SFB-F2304, and P19425-B13; H.S.) and (grant P19474-B13) (A.E.-B.).
Authorship
Contribution: F.G., S.T., J.J., C.V., S.R., D.K., S.K., and C.M. performed experiments and analyzed data; S.K., C.B., and A.E.-B. analyzed data; F.G., S.K., and H.S. wrote the paper and designed research; and H.S. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Herbert Strobl, Institute of Immunology, Medical University Vienna, Lazarettgasse 19, A-1090 Vienna, Austria; e-mail: herbert.strobl@meduniwien.ac.at.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal